Original Articles: 2021 Vol: 13 Issue: 9
The QbD Influence on the Pharmaceutical Drug Development
Vaghela N*, Mehta B
Department of Quality Assurance, Smt. S. M. Shah Pharmacy College, Mhemdabad, Gujrat, India
Abstract
Quality is a backbone of the pharmaceutical industry and the main aim of the pharmaceutical industry is to supply a drug product with ample quality, safety and efficacy. Quality by design allows for a systematic approach to drug development that is intended to improve quality by using analytical and risk-management methodologies and sound science for the design, manufacturing, development based on scientific principles has been improve in the developing quality products with a high level of reproducibility. QbD is an approach to method development, controlling all stages of the analytical procedure life cycle. Quality by design deals with the increase in the parole of providing safe and effective drugs to customers and promises to improve the efficiency of product eligibility for use. The racy framework provided a lower in risk management. QbD gives higher belief in that product have high therapeutic and provide believe that product does not have any types of microorganism growth. To ensure the product quality, safety and efficacy we can directly Target to the Drug Profile (TDP). The quality target to the product profile that acknowledge the Critical Quality Attributes (CQAs) of the drug product. The ICH Q10 is related to pharmaceutical quality system, ICH Q9 is to related quality risk management, ICH Q8 is related to pharmaceutical development. These ICH guidelines provide the complete procedure for applying analytical QbD procedure.
Keywords
Process design, Pharmaceutical development, Quality risk management, Conventional process
Abbreviations
QbD: Quality by Design; PQLM: Product Quality Lifecycle Implementation; ICH: International Conference on Harmonization; FDA: Food and Drug Administration; TPQP: Target Product Quality Profile; CQA: Critical Quality Attributes; PAT: Process Analytical Technology
Introduction
Quality by Design (QbD) is the concept was first developed or outlined by the quality promoter Dr. Joseph M juran. He believed that quality should be planned into product. QbD is unfolded the increase the assurance of safely and effectively supply a drug to the patient. And also gives promise to significantly improve manufacturing quality performance. Quality means “standard or suitability for the intended use”. It contains such attributes as the potency, quality and purity [1]. In 2002 FDA notify new invents for risk management to modernize the FDAs regulation for maintain good pharmaceutical quality as well as build up new regulatory framework focusing on QbD, quality maintaining system, risk management. The QbD concept is considered by FDA. The QbD concept generated by International Conference on Harmonization (ICH), to guide the quality i.e. ICH Q8 and ICH Q9. The pharmaceuticals industry has highly improved morbidity rates, but potency, delivery rate and other properties of drug have been reduced by various elements of risk to patients. Currently a QbD approach has been successfully implemented in the common formulation development. According to ICH Q8 guidelines, QbD is defined as, “A systematic approach to development that begins with predefined objectives and emphasizes product, process understanding and process control, based on sound science and quality risk management.” Formulation parameters (independent variables) affect the product’s characteristics and useful in optimization single variables in way to monitor the behavior of dependent variables in producing the optimized product, under the given pool of conditions [2]. Variables are included in QbD to ease the final good formulation of product (Figure 1).
The goal of Product Quality Lifecycle Implementation (PQLM) is highly emphasized on “how to” implement guidance of QbD according to ICH Q8, ICH Q9 and ICH Q10. Quality by design has approaches like scientific, systemic and risk-based for pharmaceuticals to develop the best optimized pharmaceutical dosage form. QbD ensure the predefined product quality objective in designing and development of formulation and manufacturing processes. QbD also useful in identification and characteristics of drug [3].
The concept of QbD is not a new concept but it is still a sound science and tool in the pharmaceutical industry.
The QbD also capable to found that how drug product is developed, formulated, discovered, and regulated. Quality risk management and knowledge management are the first enablers of QbD [4]. Which provide a critical role in the development and in the implementation of QbD.
So many implementation details are not discussed in the guidelines or any document. This paper describes the objective of QbD and development in pharmaceutical industry and details in the concept and implementation tools.
Background of Quality by Design
Quality by design concept firstly discovered by Juran the concept of QbD is mention in the ICH Q8 guidelines “for the identification of quality it cannot be tested in the product”. It defines the quality by design as “QbD is systematic approach to design a formulation of predefined quality and its manufacturing process to continually and consistently delivering intended effect of the final product. The data collected from the pharmaceutical development studies and formulation experience, applied for logical understanding for design space and its specification and process control, which is based on sound science and quality risk management” and the annex of ICH Q8 it provides further clarification of concept outlined in the core guideline the principle of QbD [5].
Since long time quality by design have been used for different product development just like design of experiment etc. Failure Modes and Effects Analysis (FMEA) was evaluated in 1950s for study problems that arise from go wrong of military system. For further study of that FMEA developed software in 1990 which is developed in the QbD methodology [6]. Recently ICH Q8 gives details of the principle of QbD and explains the key concept which is described in ICH Q8.
QbD Include the Key Concept and Elements During Pharmaceutical Development
• Describe target product quality profile.
• Development and designing of product and manufacturing process.
• Identification of Critical Quality Attributes (CQA), variability sources and parameters of process.
• Finally controlling manufacturing process for consistently get quality over time.
Certain key concept of QbD: The “target product quality profile”
The TPQP is defined as “A prospective summary of the quality characteristics of a drug product that ideally will be achieved to ensure the quality, taking into safety and efficacy of product”.
Recently the uses of TPP in development of the clinical and commercial decision making, planning, regulatory agency interaction, risk management, has started [7].
Process design and development: Process design is a primary stage of the process development which an outline of the commercial manufacturing processes is involved, including the scales of manufacturing.it should include all the factors that essential for design of the process, like equipment, facility related to process, material transfer, and manufacturing variables. Another factor must be included like QTPP and CQAs.
Drug excipient and drug substance properties: To get the drug product quality consistently specified in the label, the drug substance should be thoroughly characterized about its physical, biological, chemical, and mechanical properties such as stability, particle size, flow properties, polymorphism, etc [8].
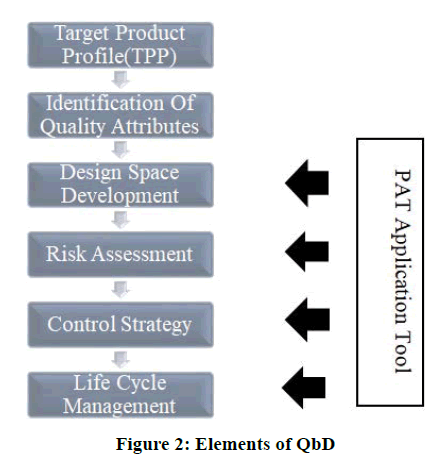
Technical elements of the QbD process it contains following technical elements as shown in Figure 2.
• Critical Quality Attributes (CQAs) are connected to the clinical relevance because of their effect on safety, efficacy, or reproducible therapeutic effect.
• Clinically relevant critical quality attributes which is linked with Critical Process Parameter (CPPs), either directly or indirectly.
• Mostly CPPs, which control the clinically relevant critical quality attributes enabled by monitoring or by Process Analytical Technology (PAT).
QbD for pharmaceutical products and its important steps in its implementation:
• Firstly, identify Critical Quality Attributes (CQAs) i.e. physical, biological, chemical, microbiological characteristic.
• Describe and generate process design space i.e. performing risk assessment linking material attributes and process parameters to critical quality attributes.
• Establish design space link between independent, CQAs and parameter of process
• Define the controlling methodology as planned set of control derived from recent product and process understanding that product quality and performances of process.
• Process validation involves manufacturing process.
• Process monitoring [9].
Ich Guidelines
Pharmaceutical Development (Q8)
This guideline provides pharmaceutical development of drug product guideline as explained in the module 3 of the J. Chem. Pharm. Res., 2021, 13(9): 21-26 CTD. While clinical trial stage of the product the guideline of ICH Q8 are not applicable for the data. ICH guideline is suitable for various product but it can only applied after taking due approval from the regulatory authorities. The annex of ICH Q8 it provides further clarification of concept outlined in the core guideline the principle of QbD [10]. This annex is not to generate new standards.it explains the tool and concept and its application by applicant in design space, outlined in the parent Q8 document.
Quality Risk Management (Ich Q9)
This guideline is mainly concerned with the principle and example of quality risk management. It may regarded as document having answer to all questions related to quality of product which is parameter including the development, processing, designing, assessment, distribution, submission process throughout the lifecycle of product also include the biotechnological and biological product, it includes vehicles/solvent, additives, excipient, material used for packaging and labelling.
Risk assessment is important step require for the ensuring of effective risk management.
Pharmaceutical Quality System (ICH Q10)
This guideline describes the quality of drug substances and its product, includes biological and biotechnological product, throughout the life cycle of the drug products. Each stages of product life cycle it should be validated by using elements of Q10 in an appropriate manner [11]. The principle of this guideline is used for the recognized the difference, and different objective at different stage (Figure 3).
Target Product Profile
Food and drug administration give guideline for defining Target Product Profile (TPP). As per guideline “The Target Product Profile (TPP) is totally corelated to the drug development program that gives the knowledge of drug during development. The TPP is use to develop link for drug development activities, drug labelling”.
As per the ICH Q8 guidelines the pharmaceutical development includes “recognition of critical quality attributes of the drug product with consideration of its intended use as well as route of administration”. Hence, it becomes essential to consider the intended usage and route of administration [12].
Critical quality attribute: The identification of the Critical Quality Attributes (CQAs) typically takes place during the first stage of the process validation which is process design space.
As per the ICH guideline define as quality attribute that comprise of chemical, biological, physical or microbiological property and its desired that these attributes should be controlled to give assurance that product attain desired stability, safety, efficacy, performance as well.
All the quality attributes are target elements of the drug product and it should be achieved through:
• Appropriate formulation and process design development
• A good quality management system (Table 1).
| Process | QbD manufacturing | Conventional process |
| Uses | Controlled manufacturing | Fixed manufacturing process |
| Consistent finished product | Variables in end product | |
| Variable starting material | Variables in starting materials |
Table 1: Conventional versus QbD manufacturing process
Prior Knowledge to Help Assess CQAS
• Technology transfer
• Product history and manufacturing history
• Pharmaceutical development studies
• Raw material testing data
• Stability reports
• Suppliers and contractors
• Technical investigation
• Internal and vendor audits
• Complaint reports; adverse event reports; recalls
Qbd application and benefits: QbD fundamentally links patient requirements to drug product and then drug substance. It is used to understand product specific requirements, which can be supported by GMP.
QbD normally starts in development and progresses through to manufacturing, with the intent of producing a control strategy for commercial-scale production, sometimes, say, with a legacy product, QbD may start with an existing manufacturing process, for example, where a rich history of product and process knowledge is available [13].
QbD can be applied to small and large molecules, to drug substance and drug product, to vaccines, to combination products, to all or parts of a process, to novel drugs or to generics. It can be used by leading companies, by contract research or contract manufacturing companies or ‘virtual’ companies. QbD can be applied for various analytical methods like:
• A hyphenated technique like LC-MS
• Dissolution studies.
• To biopharmaceutical processes.
• Advanced technique like UHPLC, mass spectroscopy, capillary electrophoresis.
• Analysis of genotoxic impurities.
Advantages of QbD:
• Sources of variability can be better controlled.
• It provides a space for the invention of new techniques by continuous improvement throughout the lifecycle.
• Improve understanding of the knowledge space.
• Good regulatory compliancy movement within “analytical design space” are not considered a change in method.
• Minimize the deviations and costly investigation.
• Eliminate batch failure.
• It allows for continuous improvement in product and manufacturing processes.
• Improves interaction with regulatory authority and instead of process level they deal with science level.
Software used for QbD: It includes,
• Minitab®
• Statistica®
• JMP®
• Un-scrambler®
• Design Expert®
• MODDE®
This software provides support for chemo-metric analysis through multi-variant technique like PCA, PLS, MNLRA, etc.
Discussion
Recently the all researcher utilizes the QbD as an important tool for getting the good quality. QbD is an integral part of modern research in pharmaceutical industry. QbD can be applied to the development of analytical methods. Critical analytical factors are identified in an approach that parallel which is described for process development in ICH Q8 and Q9. All the concepts of International Conference on Harmonization (ICH) guidelines they are directly related to quality risk management, pharmaceutical quality system, product development is important for processing a pharmaceutical process in QbD. So, we can say that QbD is an important tool in the pharmaceutical research to simplify the comprehension of the product or process as well as to realize the excellent and economic product.
Conclusion
QbD is a way to deal with strategy improvement, controlling all phases of the insightful technique life cycle. Quality by configuration manages the expansion in giving protected and powerful medications to clients and vows to work on the effectiveness of item qualification for use. The scandalous structure gave a lower in hazard the board. QbD gives higher faith in the drug production, have high efficacy. To guarantee the item quality, efficacy and adequacy we can directly focus to the Target Drug Profile (TDP).
References
- Sandipan. Int. J Pharm Biomed Res. 2012: 3(2), 100-108.
- Drennen III JK. J Pharma Innov. 2008: 2, 65-66.
- Yu LX, Raw A, Lionberger R. J Generic Med. 2007: 4, 239-248.
- Lawrence X. J Chem Pharma. 2009.
- Berridge J. J Chem. 2007.
- Singh B, Beg S. J Pharm. 2013: 22, 72-79.
- Doherty G, Martha JB. J Chem Pharm. 2002: 1-32.
- Nasr M. J Pharm. 2004.
- Juran JM. J Chem Pharma. 1997.
- Sansipan. Int J Chem. 2004.
- Woodcock J. J Chem. 2004: 1-3.
- Juran JM. Int J Pharma.1992.
- Angshetti JN, Deshpande M, Arote R, et al. Arab J Chem. 2014.