Original Articles: 2022 Vol: 14 Issue: 2
Synthesis, Characterization and Study of New Mesogenic Compound
- Corresponding Author:
- Dushad Ram
Department of Chemistry,
College of Education for Pure Science,
Basra University,
Basra,
Iraq
Received: 07-Mar-2022, Manuscript No. JOCPR-22-012; Editor assigned: 09-Mar-2022, PreQC No. JOCPR-22-012 (PQ); Reviewed: 23-Mar-2022, QC No. JOCPR-22-012; Revised: 28-Mar-2022, Manuscript No. JOCPR-22-012 (R); Published: 07-Apr-2022, DOI:10.37532/ 0975-7384-22.14.014.
Abstract
Novel compound of rod-like molecules with benzothiazole-aromatic system having an schiff base and ester linkages were synthesised. The structure of synthesised compound was confirmed by using physicochemical techniques such as 1H-NMR, mass spectra and FT-IR spectra. Differential Scanning Calorimeter (DSC) and Polarizing Optical Microscopy (POM) have been employed to verify their liquid crystalline behaviours and transition temperatures. The compound showed nematic phase only
Keywords
Benzothiazole, Liquid crystal, Schiff Base, Resonance Delocalisation, Mesomorphic.
Abbrevations
DSC: Differential Scanning Calorimetry; POM: Polarised Optical Microscope; NMR: Nuclear
Magnetic Resonance
Introduction
Liquid crystallinity or mesomorphism is a unique state of matter intermediate between a crystalline solid and a regular isotropic liquid. The phenomenon is usually exhibited by long, rod-shaped molecules which contain dipolar groups. It permits molecules to orient along the long axis. Such compounds behave as anisotropic liquids and are birefringent [1].
In chemical research, attempts to correlate compound properties with molecular structure are common. A familiar example is the systematic variation of substituent groups on a physiologically active parent compound to determine how these changes affect activity. Liquid crystalline molecular systems [2-4] have been investigated similarly to ascertain the effects of structural variations on the temperature range over which the mesophase is stable [5].
Interestingly, heterocyclic materials are vital for many optical applications, such as liquid crystal displays [6,7], optical organic transistors [8,9] and biomolecules fluorescent probes [10,11]. These properties are due to their ability to create linear or lateral dipoles and geometrical shapes, which are essential for their optical and electronic properties [12-14]. The presence of electronegative heteroatoms (O,N,S) can significantly impact bond angles, resonance delocalisation, and molecular structure [15-20].
Liquid crystal molecular structures are constructed based on the factors and principles of anisotropic mesogenic shapes. Many Schiff base/ester liquid crystals based on two or three-ring compounds have been investigated. Their optical behaviours were studied to understand the relationship between mesogens’ molecular geometry and mesomorphic properties [20-24]. Moreover, low molar mass liquid crystal compounds comprising a single mesogenic unit [25,26] exhibit significantly different behaviour than molecules having two or more mesogenic groups [27-29]. Thus, adding new mesogens or aromatic rings and different terminal substituents (compact group or alkoxy/alkyl chains) impacts molecular geometry and offers wide thermal stability ranges for the designed materials [30].
Different linkage units, such as azo, ether, ester, and amine have been introduced between aromatic rings to connect and increase the length of the long axes of calamitic molecules [31,32]. The ester linkage unit is widely used in calamitic L.C due to its stability and easy synthesis. Also, it contributes to a lamellar order and the formation of smectic phases due to carbonyl group polarizability. The imine linkage units have been widely used in the large thermotropic mesogens class. Their use in calamitics is required to synthesise imine-based ortho-metallated mesogens that exhibit physical properties differing from traditional liquid crystals due to their metal centre's redox properties and polarizability [33,34]. The thermotropic mesomorphism of calamitic L.C is largely influenced by the nature of terminal alkyl, alkoxy, and other chains.
Recently, Naoum, et al. synthesised, characterised, and studied the exchange effect of a terminal substituent. They found that most synthesised compounds having terminal electron-donating substituents exhibited purely nematogenic behaviour depending on the alkoxy chain length [35,36].
However, compounds bearing terminal electron-withdrawing substituents showed dimer and purely smectogenic (SmA) character for lower and higher homologues, respectively [37,38]. Sardon, et al. reported four azo-ester mesogens synthesised with a lateral methyl group, differing based on terminal substituents -H, -Cl, -Br, and -CN. The compound with -H terminal substituent displayed nematic behaviour with a narrow mesophase range, while electron-withdrawing groups terminated compounds exhibited mesophase behaviour with a wider nematic range [39]. Hager, et al. synthesised three new groups of 2-hydroxy pyridine ester-based liquid crystals named 5-[2-4-substituted phenyl)diazenyl]pyridine-2-yl-4 alkoxy benzoate. Each group differs at the terminal polar substituent X (CH3O, Cl and H). Thermal stability ranges and the type of mesophases observed for the investigated compounds mainly depended on the polarity, dipole moment, and charge distribution [40].
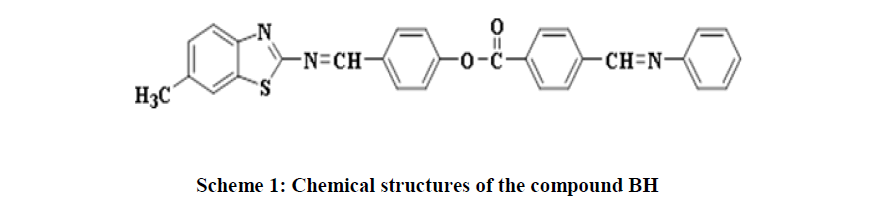
Karim, et al. synthesised a series of azo-ester linked mesogen containing liquid crystalline acrylate compounds having different terminal groups (-F, -Cl, -Br, -OCH3, -OC2H5 and -OC3H7). All compounds showed enantiotropic liquid crystal phase behaviour, and terminal substituents greatly influenced the mesophase formation. Alkoxy (-OCH3 -OC2H5, and -OC3H7) substituted compounds exhibited greater mesophase stability than halogen (-F, -Cl, and Br) terminated derivatives [37]. In the present work, the goal was to synthesise new BH and investigate their mesophase behaviour via Differential Scanning Calorimetry (DSC) and Polarised Optical Microscope (POM) as shown in Scheme 1.
Methodology
General
Infrared spectra were recorded as KBr pellets on a Buck-M500 spectrometer (Buck Scientific, USA). 1H and 13C-NMR spectra were recorded on a Bruker instrument using CDCl3 as solvent and TMS as internal standard. Transition temperatures were determined using a Perkin Elmer-Pyris DSC-8000 differential scanning calorimeter with a heating rate of 10°C min-1. The phase transitions were observed with a LeitzLaborlux 12 Pol optical microscope with polarized light in conjunction with a Leitz 350 hot stage (Germany) equipped with a Vario-Orthomat. Mass spectra were recorded on an Agilent technologies 5957C spectrometer.
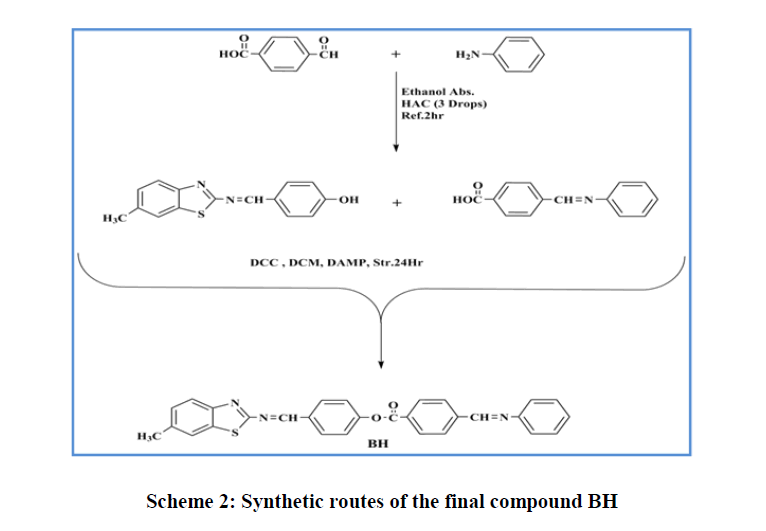
Synthesis of Schiff bases: One molar equivalent of the appropriately substituted aniline was added to a solution of the appropriate benzaldehyde in ethanol (1:1) to which a few drops of acetic acid were added according to the earlier reported method [41,42]. The mixture was heated under reflux for 2 h and the solid obtained upon cooling was filtered and twice recrystallized from ethanol to give pure compounds (Scheme 2).
Synthesis of 4-(methylthio and methoxy)benzoic acid: 4-Methylthio-benzoic acid or 4-hydroxy-benzoic acid was synthesised according to the literature procedure [43] (Scheme 2) by refluxing 4-mercaptobenzoic acid or 4-hydroxy-benzoic acid, 1-bromomethyl and potassium carbonate in ethanol for 24h. At the end of the reaction, solvent was evaporated and water was added and the aqueous solution was heated to boiling and then cooled to room temperature. A few drops of conc. HCl were added until a solid precipitated. The solid was filtrated and washed with water to afford the target compound (Scheme 2).
Synthesis of esters: Molar equivalents of acids and phenols (0.01 mol) were dissolved in 25 ml of DCM. To the resulting mixture, (DCC, 0.002 mol) and (DMAP, 0.002 mol), were added and the mixture was kept stirring at room temperature for 6h following the reported procedure [44,45]. The precipitated solid (by product) was filtered off and the filtrate was evaporated and the residue was twice crystallised from ethanol (Scheme 2).
4-(((6-methylbenzo[d]thiazol-2-yl)imino)methyl)phenyl 4-((phenylimino) methyl) benzoate (BH): Chemical Formula: C29H21N3O2S; yellow solid; yield 45%; 1H-NMR spectrum (CDCl3, 499MHz): δ (ppm) 2.50(s, 3H, CH3), 7.31-8.48ppm(m,16H,Ar-H), 8.56(s, 1H, CH=N ), 9.16(s, 1H, CH=N); 13C-NMR (CDCl3, 125MHz): δ (ppm) 21.76 (CH3),121.09-130.84ppm(Ar-C), 160.37 (CH=N), 164.55 (CH=N), 167.48 (C=O), 174.09 (C=N). IR(cm-1): 3032- 3059 (C-H aromatic), 2850-2924 (C-H aliphatic), 1732 (C=O), 1622 (CH=N), 1600 (C=N thiazole), 1448-1587 (C=C aromatic), 1280 (C-O) ; MS (m/z) 475.3 (M+) (Figure 1).
Result and Discussion
Thermodynamic properties (enthalpy and entropy), textures, and the phase transition temperatures of the liquid crystal compound were determined using Differential Scanning Calorimetry (DSC) thermograms and Polarised Optical Microscopy (POM) connected to a hot stage. The DSC heating data and curves of the synthesised molecule (BH) are listed in Table 1.
| C→N | N→I |
|---|---|
| Comp. | B1H |
| Transition Temp.(° C) | 205.14 |
| ΔH kJ/mol | 31.29 |
| ΔS J/mol. K | 65.45 |
| Transition Temp.(° C) | 293.06 |
| ΔH kJ/mol | 2.41 |
| ΔS J/mol. K | 4.27 |
| ΔTN (° C) | 87.92 |
Note: C: Crystal, N: Nematic, I: Isotropic, ΔTN: thermal range for nematic phase
Table 1: Transition temperatures, corresponding enthalpy values (ΔH, kJ.mol-1), and entropy changes (ΔS,J/mol.K) for the compound BH
The compound BH Showed nematic phase only with thermal 87.92?C, it is due to the absence of a substituted terminal group at the end of the molecule that might enhance terminal attraction forces and the emergence of nematic phase [40].
The appearance of the nematic and smectic phase in liquid crystalline compounds depends on the ratio of the terminal and lateral attraction forces specific to the compounds. The nematic phase appears when the terminal attraction force is high, while the smectic phase appears for a lower attraction force [46]. Terminal compensating groups in molecules affect terminal attraction force. Groups with a dipole moment across the molecular axis enhance the terminal attraction forces, and the nematic phase appears. If the dipole moment is across the molecular axis, the molecules follow two arrangements to offset the repulsive forces between similar charges and maintain the parallel arrangement of the particle [46,47]. The molecules tend to be at an angle to diverge similar charges and reduce repulsion in the first order. This arrangement promotes the emergence of the smectic phase. In the second order, the particles take the staggered arrangement (finger arrangement), promoting the emergence of the nematic phase [48,49]. The presence of the benzothiazole group in all compounds has two opposite effects.
Conclusion
In this work, we have reported the synthesis and mesomorphic properties of BH, the enantiotropic nematic phase was observed throughout the BH. The first effect enhances the appearance of liquid crystalline properties by increasing molecule polarity due to the two sulphur and nitrogen atoms. The other effect increases molecular width and favours the nematic phase than the smectic phase. The compound BH exhibited nematic phase in a marble texture by heating and schlieren texture on cooling.
References
- Castellano JA, Goldmacher MJE, Barton LA, Kane JS. J Org Chem. 1968;33(9),3501-3504.
- Brown GH, Shaw WG. Chem Rev. 1957;57(6),1049-1157.
- Gray GW. Academic press. 1962.
- Saupe A. Angew Chemie Int Ed English. 1968;7(2),97-112.
- Schroeder JP, Bristol DW. J Org Chem. 1973;38(18),3160-3164.
- Demus D, Goodby J G. 1998.
- Bushby RJ, Lozman OR. Curr Opin Colloid Interface Sci. 2002;7(5-6),343-354.
- Savagian LR, Österholm AM, Ponder JF, Barth KJ, et al. Adv Mater. 2018;30(50),1-6.
- Paterson AF, Singh S, Fallon KJ, et al. Adv Mater. 2018;30(36),1-33.
- Chin J, Kim HJ. Coord Chem Rev. 2018;354,169-181.
- Dias GG, King A, De Moliner F, Vendrell M, et al. Chem Soc Rev. 2018;47(1),12-27.
- Lehn JM. Science. 2002;295(5564),2400-2403.
- Titov VV; Pavlyuchenko AI. Chem Heterocycl Compd. 1980;16(1),1-13.
- Zaki AA, Ahmed HA, Hagar M. Mater Chem Phys. 2018;216,316-324.
- Lehmann M, Kestemont G, Aspe RG, et al. Chem-A Eur J. 2005;11(11),3349-3362.
- Takase M, Enkelmann V, Sebastiani D, Baumgarten M, et al. Angew Chemie-Int Ed. 2007;46(29),5524-5527.
- Hagar M, Ahmed HA, Alhaddad OA. Liq Cryst. 2019;46(9),1440-1451.
- Ahmed HA, Hagar M, Alaasar M, Naoum M. Liq Cryst. 2019;46(4),550-559.
- Ahmed HA, Hagar M, Aljuhani A. RSC Adv. 2018;8(61),34937-34946.
- Ahmed HA, Hagar M, Alhaddad OA. Crystals. 2019;9(3).
- Huang CC, Hsu CC, Chen LW, Cheng YL. Soft Matter. 2014;10(46),9343-9351.
- Segura JL, Mancheño MJ, Zamora F. Chem Soc Rev. 2016;45(20),5635-5671.
- Gowda A, Jacob L, Joy N, Philip R, et al. New J Chem. 2018;42(3),2047-2057.
- Hagar M, Ahmed HA, Saad GR. Liq Cryst. 2018;45(9),1324-1332.
- Imrie CT, Henderson PA. Curr Opin Colloid Interface Sci. 2002;7(5-6),298-311.
- Imrie CT, Henderson PA. Chem Soc Rev. 2007;36(12),2096-2124.
- Ahmed HA, Hagar M, El-Sayed TH, B. Alnoman R. Liq Cryst. 2019;46(7)1-11.
- Hagar M, Ahmed HA, Saad GR. J Mol Liq. 2019;27,266-273.
- Ahmed HA, Hagar M, Saad GR. Liq Cryst. 2019;46(11),1611-1620.
- Ahmed NHS, Saad GR, Ahmed HA, Hagar M. RSC Adv. 2020;10(16),9643-9656.
- Selvarasu C, Kannan P. J Mol Struct. 2016;1125,234-240.
- Bubnov A, Kašpar M, Hamplová V, Dawin U, et al. Beilstein J Org Chem. 2013;9,425-436.
- Serrano JL. 1996;3(527),29296-9.
- Donnio B, Guillon D, Deschenaux R. 2003.
- Naoum MM, Fahmi AA, Ahmed NHS, Saad GR. Liq Cryst. 2015;42(11),1627-1637.
- Naoum MM, Fahmi AA, Ahmed NHS, Saad GR. Liq Cryst. 2015;42(9),1298-1308.
- Karim MR, Sheikh MRK, Yahya R, Mohamad Salleh N, et al. Liq Cryst. 2016;43(12),1862-1874.
- Racané L, Mihali? Z, Ceri? H, Popovi? J, et al. Dye Pigment. 2013;96(3),672-678.
- Niori T, Sekine T, Watanabe J, Furukawa T, et al. J Mater Chem. 1996;6(7),1231-1233.
- Hagar M, Ahmed HA, Saad GR. Liq Cryst. 2020;47(1),114-124.
- Al-Hamdani UJ, Jassem AM, Dhumad AM, Al-Shlshat S. Liq Cryst. 2021;00(00),1-14.
- Al-Hamdani UJ, Al-Ibrahim AG, Abbo HS, Titinchi SJJ. Mol Cryst Liq Cryst. 2015;607(1),13-22.
- Al-Hamdani UJ, Abbo HS, Shaheeb EH, Titinchi SJJ. Liq Cryst. 2019;46(15),2291-2300.
- Al-Hamdani UJ. Int J Mol Sci. 2011;12(5),3182-3190. [Cross Ref]
- Al-Hamdani UJ, Abbo HS, Al-Jaber AA, Titinchi SJJ. Liq Cryst. 2020;47(14-15),2257-2267.
- Al-Hamdani UJ, Gassim TE, Radhy HH. Molecules.2010;15(8),5620-5628.
- Gray GW. Academic press.1962;1(3),303-379.
- Collings PJ, Hird M. Taylor Fransis. 2004;314
- Ha ST, Koh TM, Win YF, Sastry SS. Liq Cryst. 2013;40(8):1016-1023.