Original Articles: 2021 Vol: 13 Issue: 10
Statin Microspheres for Angiogenesis and Osteogenesis Activity: Review
Abstract
Statins are widely used for the hyper-lipidemic condition. Besides the lipid lowering level, it plays a crucial role in osteogenesis and angiogenesis by inducing the Bone Morphological Protein (BMP-2). It can be delivered locally by new approaches like nanostructured microspheres and tissue engineering approaches. It helps to improve the therapeutic effects of statin in osteogenesis and angiogenesis activity. These approaches help to overcome the factors of statin therapy. In this review, various methods like nanostructured microspheres, injectable hydrogels and TERM methods are discussed. The clinical studies and in vivo studies confirm the beneficial effects of the statin groups. This helps to develop the delivery system and tissue engineering technologies in future aspects.
Keywords
Angiogenesis activity; Nanospheres; Poly-microspheres
Introduction
Statins are HMG-CoA reductase inhibitors which help in lowering blood cholesterol [1]. But statins have ability to modulate inflammation, enhance osteo-induction, osteogenesis and angiogenesis. It also inhibits osteoblast apoptosis and osteo-clastogenesis. It exerts their anabolic effects on bone by differentiating mesenchymal cells to osteoblast via up-regulating BMP-2. Statin induce angiogenesis by promoting endothelial cell migration,maturation and survival.
Different type of statin has been used in clinical studies such as simvastatin, atorvastatin, lovastatin, pravastatin, cerivastatin, fluvastatin, pitavastatin, rosuvastatin. Based on the clinical studies, liphophilic statins particularly simvastatin, atorvastatin and lovastatin seem to be most effective statin types for osteoporosis prevention and fracture healing [2].
Angiogenesis is the formation of new blood vessels from pre-existing ones, is stimulated after fracture by the local production of numerous angiogenic growth factor [3]. Vascularization also plays a major role in bone tissue engineering, which aims at developing bone substitutes to replace tissues losses due to trauma, tumour or surgery.
There is a close connection between the angiogenesis and osteogenesis during the process of bone repair. Statin enhance angiogenesis through up regulation of gene expression of Vascular Endothelial Growth Factor (VEGF) and basic Fibroblast Growth Factor (FGF-2) which induce BMP-2 expression and stimulate osteoblast differentiation indirectly [4].
The most side effects of statin are in close relation with higher systemic administrative doses. The major factors such factors such as drug interaction, hepatic dysfunction, renal insufficiency, genetic variant, elderly hypertension and obesity are the increased risk of statin related side effects [5].
There are some of the direct carrier for statin which is targeted for angiogenesis and osteogenesis activity.
• Nanostructured Calcium Phosphate Porous Microspheres (CAP)
• Mesophorous Hydroxyapatite Microspheres (MHM )
• Poly (Lactide-co-Glycolic Acid) [PLGA] microspheres
• Nano-spheres
• Injectable hydrogel system
• Tissue Engineering and Regenerative Medicine (TERM)
Nano-structured materials are promising drug carriers owing to their advantages including large specific surface area, high drug loading capacity and controlled drug release behaviour [6]. Furthermore, ACP has a good biodegradability and an ability to promote osteoblast adhesion and osteo-conductivity.
Hybrid hydrogels are highly hydrated polymeric network, either physically or covalently cross linked together and or with drugs, nanoparticles or nanostructure and it have attracted increasing as TERM scaffolds for the sustained release of drug. It has high biocompatibility, easy loading of drug and chemicals, easy implantation and minimal invasive process. TERM scaffolds are commercial availability, controllable biodegradation, superior drug release and they do not extensive and expensive process [7].
Literature Review
Methodology
Preparation of nano-structured ACP microspheres: Amorphous Calcium Phosphate (ACP) microspheres with a porous and hollow structure have been prepared using an aqueous solution containing cacl2 as a calcium source, adenosine triphosphate disodium salt (Na2ATP)as a phosphorous source in the presence of a block co-polymer methoxyl Poly (Ethylene Glycol)-block-poly(D,L-lactide) (mPEG-PLA) by the microwave assisted hydrothermal method. Statin is dissolved with ethanol and drug is loaded in the CAP microspheres [8-11].
Preparation of MHM microspheres: MHM were consisted of hydroxyapatite nano sheets/nano rods that were hierarchically assembled into nanostructured mesoporous microspheres, and it is synthesized by fructose 1,6-bisphosphate tri-sodium salt as an organic phosphorous source [12]. Then the statin is dissolved in ethanol and loaded in MHM microspheres.
PLGA microspheres by electro-spraying method: Electro-spraying solution containing PLGA and statin were prepared by adding PLGA and drug to the methylene chloride followed stirring vortex mixer until clear solution were formed. Spraying solution was prepared based on the total solute content to obtain the micro-particle.
Preparation of statin loaded PLGA Nanospheres (NSP): It is prepared by using O/W single emulsion/solvent diffusion technique. PLGA was dissolved in acetone, then the drug was added and the mixer is sonicated in ultrasonic wave cleaner to produce the oil phase. This solution was then added to PVA-ethanol solution and sonicated again to form O/W emulsion [13-15]. The resulting NSP suspension was centrifuged to remove residual solvent. Then washed three times in distilled water and it is frozen in freeze dryer. The preparations were stored at -80°C until further use.
Preparation of statin loaded injectable microsphere hydrogel system: Statin loaded PLGA micro-spheres, gelatine, glycerine and deionized water were mixed using ultrasonic agitation at room temperature to form a homogeneous hydrogel, which was loaded into a sealed syringe. Hydrogel were sterilized using cobalt-60 irradiation and preserved at 4°C for later use [16].
TERM and local delivering of statin: In TERM methods, statins can be combined with scaffolds, microspheres,hydrogels or a combination of them as a sustained delivery system. Based on the type of carrier, the carrier-statin construct can be directly injected in the injured area by a single injection or can be surgically implanted.
Evaluation
Particle size distribution: The particle size distribution for formulation containing statin microsphere was determined by Malvern master-sizer S-2000. For this purpose, microspheres were added in to water containing Tween 80 and homogenously dispersed. All measurements were performed [17-20]. The statistical comparisons were done by using IBM SPSS statistics software with the one way ANOVA. The p value was set as 0.05 and the post hoc comparisons were done by dunnett T3 test.
Encapsulation efficiency: 10 mg of microspheres have been accurately weighed and transferred into a vial containing 1 ml of dichloromethane. The vial is vortexed for 2 minutes for the destruction of microsphere structures. Afterwards, dichloromethane is evaporated until dryness and 1 ml of methanol is added into the vial for the solubilisation of statin microsphere [21-23]. The vial is vortexed for 2 minutes and kept in the ultrasonic bath for an additional 2 minutes to recover the total amount of statin microsphere existing in the samples. This solution is directly filtered through 0.45 μm PTFE syringe filter and analyzed with the developed HPLC method. The encapsulation efficiency was determined and results were expressed with standard error of means.
Scanning electron microscopy: The surface characteristic of the microsphere formulation were investigated with scanning electron microscopy. The samples were mounted on aluminium stubs and sputter-coated with goldpalladium under an argon atmosphere before analysis [24].
Fourier Transform Infrared Spectroscopy-FTIR: Fourier transform infrared analysis was conducted to verify the encapsulation of statin inside the microsphere [25-27]. The samples were scanned in the IR and the background spectrum was subtracted from the IR signal.
Thermo-gravimetric and differential scanning calorimetry analysis: Simultaneous thermo-gravimetric analysis and differential scanning calorimetry were performed using Q600 SDT system in order to verify the thermal stability of the samples. Thermo-gravimetric and differential scanning calorimetry curves were obtained using platinum crucibles with about 1 mg of samples, under nitrogen atmosphere and heating rate of 10°C/minutes [28].
In vitro release studies: In vitro release tests were carried out in a horizontal shaker.10 mg microspheres were accurately weighed and added into 30 ml of phosphate buffer in flasks. Flasks were fixed in a horizontal shaker water bath at 50 rpm. At pre-determined time intervals, 1 ml of sample was withdrawn and replaced with a fresh medium immediately for maintaining sink conditions [29-31]. The sample was filtered through 0.45 μm cellulose acetate filters and analyzed by using HPLC method. For each formulation, in vitro release characteristics are investigated over 3 replicates and the release kinetics were analyzed by graph pad software.
Cytotoxicity of drug loaded microspheres: To determine cytotoxicity of the statin loaded microspheres they were sterilized under UV light for 45 minutes and homogenized into cell culture medium with vortex for 2 minutes. Then sterile microspheres were applied with the concentration of 1 mg/ml on L-929 and B-35 cell cultures and cultured for 24 hours and 48 hours [32-35]. After specific time culture period, colorimetric MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay was performed to investigate cytotoxicity of microspheres. At each culture time drug treated cells were incubated with 5 mg/ml of MTT reagent for 3.5 hours. After that di-methyl sulfoxide was added to dissolved and measure using an ELISA micro plate reader.
Stability: The short-term stability of statin microsphere was examined by keeping the stock solutions at concentration of low and high at 37 ± 0.5°C for 8 days. For each concentration three replicates were analyzed in one analytical batch. The concentrations of statin microspheres after each storage period were related to its initial concentration as determined for the samples that were freshly prepared and possessed immediately [36].
In Vitro and In Vivo Assay of Angiogenesis
Assays for endothelial cell migration
Modified Boyden chamber assays have been used to assess endothelial cells are plated on top of a filter usually coated with a matrix protein such as fibronectin or collagen and migrate across this in response to a test angiogenic factor placed in the lower chamber.
An increase in cell motility can also be a measure of an angiogenic response, as angiogenic factors are known to stimulate cell movement. This is observed in the Boyden chamber assay but is more accurately measured using phagokinetic track assay methods because it consist colloidal gold plate act as a substrate for movement of cells [37].
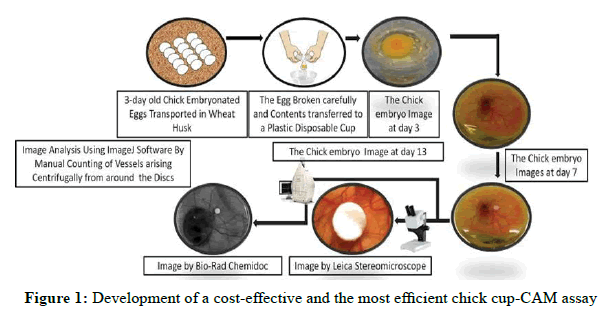
Chick Chorio-Allantoic Membrane (CAM) assay: The chick Chorio-Allantoic Membrane (CAM) assay is probably the most widely used in vivo assay for studying angiogenesis. The test substance is prepared either in slow release polymer pellets, absorbed by gelatine sponges, or air-dried onto plastic discs, these are then implanted on to the CAM through a window cut carefully in the eggshell. The lack of a nature, immune system in 7-8 days old chick embryos allows for the study of tumour induced angiogenesis. The angiogenic effects can be measured by counting the number of blood vessels in a given area using a stereomicroscope. In a variation of the CAM assay, shell-less embryos are cultured in petri dishes prior to applying the test substance this allows for the quantification of blood vessels over a wider of the CAM [38].
However, the CAM itself has a well-developed vascular network, thus making it difficult to distinguish new capillaries from existing ones furthermore; the 7-8 days old CAM often develops an inflammatory response to a variety of irritants, including shell dust generated when cutting the window in the shell, which can also hamper the identification of new vasculature [39]. It is usually necessary to wait for 3 days after making the window before adding the test substance, to check for any immune response.
Dorsal air sac model:The dorsal air sac model is used to examine the in vivo effect of substance against the angiogenic response triggered by cancer cells. Both sides of a Millipore ring are covered with filters and the resultant chamber carefully filled with a tumour cell suspension, this is then implanted into the performed dorsal air sac of an anaesthetized mouse. Following treatment with the compound of interest the chamber is carefully removed and rings of the same diameter placed directly upon the sites that were exposed to a direct contact with the chamber. The angiogenic response can then be assessed by counting the number of newly formed blood vessel that lie within the area marked by the ring, using a dissecting microscope [40,41].
This assay is relatively simple to perform although care must be taken not to irritate the surface upon which the chamber is placed as this may itself induce angiogenesis and hence mask those blood vessels formed due to the presence of the tumour cells.
Embryonic cell culture: Cup-CAM Method
Representative pictures of key steps of the cup-CAM method (Figure 1).
Conclusion
The present study shows that Statins inhibit the osteoblast apoptosis and promote the osteogenesis and angiogenesis by inducing BMP-2. An administration of statin enhances new bone formation by two different pathways of angiogenesis and osteogenesis. Simvastatin has been the most investigated statin type for osteoporosis and it indirectly enhances angiogenesis at high dose. From the clinical studies and in vivo studies simvastatin and atorvastatin was the most effective in compare with other statin. The other statin like rosuvastatin, lovastatin, and fluvastatin are widely used for the local delivery. It produces angiogenesis and osteogenesis activity only at low dosage. At high dose pro-angiogenic effect is reversed and side effect is expected. To overcome this microwave assisted nanostructured materials and TERM based strategies through scaffolds, microspheres and hydrogels are used. By this method, the systematic side effect has been reduced.
References
- Li L, Zhang P, Tian JH, et al. Cochrane Database Syst Rev. 2014; 12.
- Feig JE. Ann Glob Health. 2014; 80(1), 13-23.
- Stegen S, van Gastel N, Carmeliet G. J Bone. 2014; 1-3.
- Klagsbrun M, Moses MA. J Path. 1999; 6(8), 217-224.
- Colpaert CG, Vermeulen PB, Benoy I, et al. British J Cancer. 2003; 88(5), 718-725.
- https://www.news-medical.net/health/Angiogenesis-Types.aspx
- https://study.com/academy/lesson/what-is-angiogenesis-definition-factors-quiz.html#
- Reddi AH. Tissue Eng. 2000; 6, 351-359.
- Zhao C, Bi Q, Hu JT, et al. J Bio. 2013; 93(29), 2309-11
- Adams RH, Alitalo K. Nat Rev Mol Cell Biol. 2007; 8, 464-78.
- Ding GJ, Zhu YJ, Qi C, et al. J Colloid Inter Sci
- Jain RK. Nat Med. 2003; 9, 685-93.
- Potente M, Gerhardt H, Carmeliet P. J Cell. 2011; 46, 873-87.
- Ferguson C, Alpern E, Miclau T, et al. Mech Dev. 1999; 87, 57-66.
- Staton CA, Stribbling SM, et al. Int J Exp Path. 2004; 85, 233-248
- Josko J, Gwozdz B, Jedrzejowska-Szypulka H, et al. Med Sci Monit. 2000; 6, 1047-52.
- Bluteau G, Julien M, Magne D, et al. Bone. 2007; 40, 568-76.
- Bouletreau PJ, Warren SM, Spector JA, et al. Plast Reconst Surg. 2002; 109, 2384-97.
- Javerzat S, Auguste P, Bikfalvi A. Trends Mol Med. 2002; 8, 483-9.
- Saeed K, Khan I. Inst Sci Technol. 2016; 44, 435-444.
- Eroglu H, Haidar MK, et al. J Drug Del Sci Tech. 2017; 39, 455-466
- Dreaden EC, Alkilany AM, Huang X, et al. Chem Soc Rev. 2012: 41, 2740-2779.
- Thomas S, Harshita BSP, Mishra P, et al. Curr Pharm Des. 2015; 21, 6165-6188.
- Mirzadeh E, Akhbari K. Cryst Eng Comm. 2016; 18, 7410-7424.
- Avasare V, Zhang Z, Avasare D, et al. Int J Energy Res. 2015; 39, 1714-1719
- Lin Yu W, Wei Sun T, et al. J Sci Reports. 2017.
- Filipe V, Hawe A, Jiskoot W. Pharm Res. 2010; 27, 796-810.
- Li X, Liu X, et al. J Cranio-Maxillo-Facial Surgery. 2019; 47, 1147-1154
- Terukina T, Naito Y, Tagami T, et al. J Drug Del Sci Tech. 2016; 33, 136-142.
- Gaspa SR, Nogues X, Enjuanes A, et al. J cell Bio Chem. 2007;101(6), 1430-8.
- Qi C, Zhu YJ, Zhao XY, et al. Chem A Euro J. 2013: 981-987.
- Chuengsamarn S, Rattanamongkoulgul S, et al. Bone. 2010; 46(4), 1011-5
- FangSun F, Zhou H, Lee J. J Act Biomater. 2011; 7(11), 3813-28.
- Boller C, Prado MRM, Teixeira de Toledo MG, et al. Int J Current Microbio App Sci. 2015; 4(8), 231-243.
- Debnath S, Son S, Sadiasa A, et al. Int J Pharma. 2013; 443, 87-94
- Songa C, Guoa Z, Maa Q, et al. Biochem Biophy Res Comm. 2003; 308, 458-462.
- Oryan A, Kamali A, Moshiri A. J Cont Rel. 2015.
- Fukui T, MasaakiIi, TaroShoji, et al. J Bone Min Res. 2012.
- Nai M, Brahma P, Manjusha DA. Met Protoc. 2018; 1, 19
- Kureishi Y, Luo Z, Shiojima I, et al. J Bio. 2000.
- Elmadhun NYMD, Lassaletta AD, et al. J Thoracic Cardiovas Sur. 2012.