Original Articles: 2021 Vol: 13 Issue: 11
Spectrophotometric Determination of Trace Arsenic (III) in Biological Samples by Using 2,4-Di Methoxy Benzaldehyde Isonicotinoyl Hydrazone (DMBIH)
Ramakrishna Reddy K1, Viswanatha C2*, Radhakrishna N3
1Department of Chemistry, REVA University, Bengaluru, India
2Department of Chemistry, Sri Chaitanya Degree College, Chittoor, India
3Department of Chemistry, MLA College for Women, Bengaluru, India
Abstract
A rapid, simple, sensitive and selective spectrophotometric method has been developed for the determination of As (III) using newly synthesized chromogenic reagent 2,4-Dimethoxy Benzaldehyde Isonicotinoyl Hydrazone (DMBIH). As (III) forms yellow colored water soluble complex with DMBIH in the pH range 8.0-9.0. The maximum absorbance was observed at pH 8.5. The molar absorptivity and sandell’s sensitivity of As (III) at λ max 400 nm was found to be 3.7372 × 104 L mole-1 cm-1 and 0.006 μ g/cm2 .Beer’s law validity range 0.4796 to 5.5452 μ g/ml. As (III) forms 1:1 complex with DMBIH and stability constant of the complex was found to be 4.17 × 106. The first and second order derivative method has been developed. The derivative amplitudes were measured by peak height method and show maximum amplitudes at 435 nm and 449 nm respectively in alkali buffer medium. The developed method was used for the determination of As (III) in biological and water samples.
Keywords
Arsenic (III); Derivative Spectrophotometry; 2,4-Dimethoxy Benzaldehyde Isonicotinoyl Hydrazone (DMBIH); Biological samples
Introduction
Arsenic occurs naturally in the Earth’s crust in its inorganic form, trivalent (arsenate) or pentavalent (arsenate). Erosion of arsenic containing surface rocks probably accounts for a significant amount of arsenic in water supplies. It is a ubiquitous element in water, soil and sediments. The occurrence of arsenic in plants and animals generally reflects its accumulation from the environment. The presence of arsenic in drinking water has reached calamitous proportions in many parts of the world. There are numerous reports in the literature based on past and on-going experience in various countries in Asia and South America concerning the higher risks of skin, bladder, lung, liver, and kidney cancer that result from continued consumption of elevated levels of arsenic in drinking water [1]. Consumption of even low levels of arsenic over a long period can cause a multitude of diseases. The maximum permissible limit for arsenic in drinking water is 0.01 mg/L as recommended by WHO [2]. In certain areas in India, Bangladesh, China and Mongolia [3], arsenic levels in groundwater exceed 1 mg/mL. Regarding inorganic arsenic, arsenic (III) is appreciably more toxic than arsenic (V). Usually these species of arsenic in natural water are found at the trace levels [4]. There are only a few analytical techniques available, which have sufficient sensitivity and selectivity to directly determine these species of arsenic, at the trace levels in natural water. Therefore, the development of sensitive and accurate methods for speciation and preconcentration of trace amounts of As (III) and As (V) is necessary. Recently, many kinds of conventional analytical techniques, such as Hydride Generatively Coupled Plasma Atomic Emission Spectrometry (HG-ICP-AES) [5], Capillary Electrophoresis Inductively Coupled Plasma Mass Spectrometry (CE-ICP-MS) [5], high performance liquid chromatographyinductively coupled plasma mass spectroscopy [6], Electro Thermal Atomic Absorption Spectrometry (ETAAS) [7], hydride generation-atomic absorption spectrometry [8], hydride generation-atomic fluorescence spectrometry [9], cathodic stripping voltammetry [10], anodic stripping voltammetry [11], neutron activation analysis [12], photometric analysis [13], ion selective electrodes [14] and energy dispersive X-ray fluorescence spectrometry [15], have been used for the determination of low concentrations of arsenic. But all these techniques are costly and require trained staff. Recently, most of the spectrophotometric methods have been developed as an alternative for the determination of arsenic instead of conventional techniques. The hydrazones widely used as chelating agents for the separation of trace heavy metals from matrix species as well as their pre-concentration prior to determination. Among the hydrazones, are newly chelating agents which have found good application in the extraction of metal ions. The method was successfully applied for the spectrophotometric determination of As (III) in various water samples and biological samples using newly synthesized analytical reagent 2,4-Dimethoxy benzaldehyde isonicotinoyl hydrazone.
Materials and Methods
Apparatus
The absorbance and pH measurements were made on a Shimadzu UV-visible spectrophotometer (Model UV- 160A) fitted with 1.0 cm Quartz cells and Elico digital pH meter (Model LI 120) respectively. Suitable settings for derivative were as follows. The spectral band length was 5 nm, the wavelength accuracy was 0.5 nm with automatic wavelength correction and the recorder was a computer controlled thermal graphic printer with a cathode ray tube and one degree of freedom in the wavelength range 300-800 nm.
Reagents
All reagents used were of A.R grade unless otherwise stated. All solutions were prepared with double distilled water. A stock solution of Standard As (III) solution was prepared in the range of 0.2-1.0 mg/L. (E-Merck, Germany) in double distilled water in 1000 mL standard flask. As (III) working standard stock solutions were prepared freshly by appropriate dilution of the standard stock solution with double distilled water.
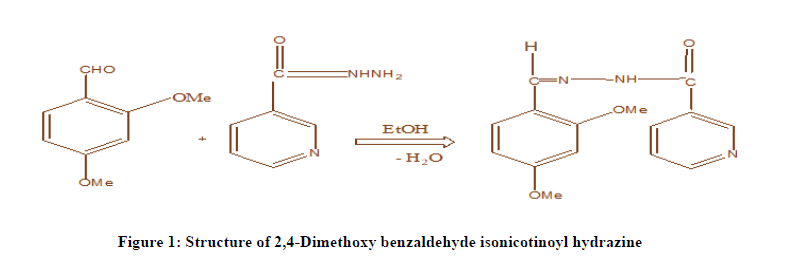
Preparation of 2,4-Dimethoxy Benzaldehyde Isonicotinoyl Hydrazone: The reagent DMBIH is prepared by the Sah and Daniels procedure. 1.6617 gm of 2,4-Dimethoxybenzaldehyde and 1.3714 gm of Isonicotynoyl hydrazide were dissolved in sufficient volume of methanol and the mixture is refluxed for four hours. The contents are allowed to cool and the product was separated by filtration. A crude sample (yield 80%) is obtained (C16H1604N2). The resultant product is recrystallized twice from hot methanol. Pure light greenish crystals of 2,4-Dimethoxy Benzaldehyde Isonicotinoyl Hydrazone (DMBIH) (m.p. 234-236℃.) was obtained. IR and NMR spectral studies characterized the compound. The mass spectrum shows that molecular ion peak at m/z 286 (M+1). The structure of DMBIH was confirmed based upon IR, NMR and mass spectral data. The structure of DMBIH shown in Figure 1.
Results and Discussion
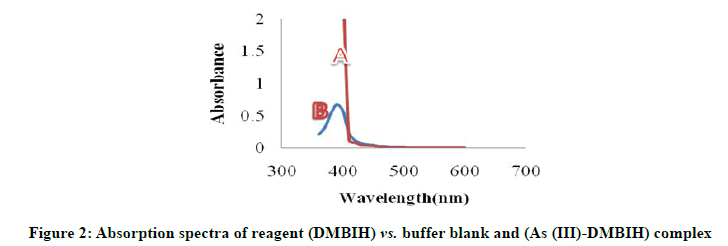
The reagent 2,4-Dimethoxy Benzaldehyde Isonicotinoyl Hydrazone (DMBIH) was easily synthesized as any other Schiff base reagent. The new chromogenic reagent DMBIH was used for the spectrophotometric method for determination of the As (III). The absorption spectra of DMBIH-As(III) complex under the optimum conditions are shown in Figure 2. The As (III)-DMBIH complex shows the maximum absorbance at 400 nm, where the reagent blank does not absorb appreciably.
As (III)=0.5 mL of 2.5 × 10-5 M
DMBIH=0.5 mL of 2.5 × 10-4 M
pH=8.5
SDBS (5%)=0.5 mL
Arsenic (III) reacts with DMBIH in basic buffer to give yellow coloured water-soluble Complex. The color reaction between arsenic (III) and DMBIH was instantaneous even at room temperature in pH range 8.0-9.0, the maximum colour intensity was observed at pH 8.5. The absorbance of Arsenic (III)-DMBIH remains constant for more than 24 hours. The effect of various surfactants such as Triton X-100 (5%), Sodium Dodecyl Benzene Sulphonate (SDBS) and Cetyl Trimethyl Ammonium Bromide (CTAB) on the absorption profiles of the system has been investigated. In presence of SDBS (5%) surfactant the complex is more stable and exhibited maximum absorbance. The results were shown in Table 1.
| SDBS | Absorbance |
|---|---|
| 0.5 | 0.775 |
| 0.8 | 0.774 |
| 1 | 0.775 |
| 1.5 | 0.776 |
| 2 | 0.774 |
| 2.5 | 0.775 |
(As (III))=2.5 × 10-5 M
(DMBIH)=2.5 × 10-4 M
pH=8.5
Wavelength=400 nm
SDBS (5%)=0.5 mL
When varying the volume of reagent DMBIH (2.5 × 10-4 M) from 0.5 mL to 3.0 mL, the constant absorbance was obtained from 0.5 mL. Therefore 10-fold molar excess of reagent is adequate for full colour development. The excess of reagent has no adverse effect on the absorbance of the complex. The order of addition of buffer solution, metal ion, and reagent has no adverse effect on the absorbance of As (III)-DMBIH complex. Beer’s law was obeyed in the range 0.48-5.54 μg/ml. The optimum concentration range of As (III)-DMBIH complex was 0.85- 4.80 μg/ml. The molar absorptivity and Sandell’s sensitivity of As (III)-DMBIH complex was obtained from the Beer’s law. The linear regression analysis of absorbance at λmax of the complex against metal ion (μg/ml) shows a good linear fit. The various important analytical characteristics of As (III) and DMBIH complex are summarized in Table 2.
| Characteristics | Results |
|---|---|
| Colour | Yellow |
| λmax (nμ) | 400 |
| pH range (optimum) | 8.0-9.0 |
| Mole of reagent required per mole of metal ion for full color development | 10 folds |
| Molar absorptivity (L.mol-1 cm-1) (e) | 3.7372 × 104 |
| Sandell’s sensitivity (μ g cm-2) | 0.006 |
| Beer’s law validity range (μ g/ml) | 0.48-5.54 |
| Optimum concentration range (μ g/ml) | 0.93-4.70 |
| Composition of complex (M:L) obtained in Job’s and mole ratio methods | 01:01 |
| Stability constant of the complex | 4.17 × 106 |
| Standard deviation for ten determinations | 0.003 |
| Relative standard deviation (%) | 0.3 |
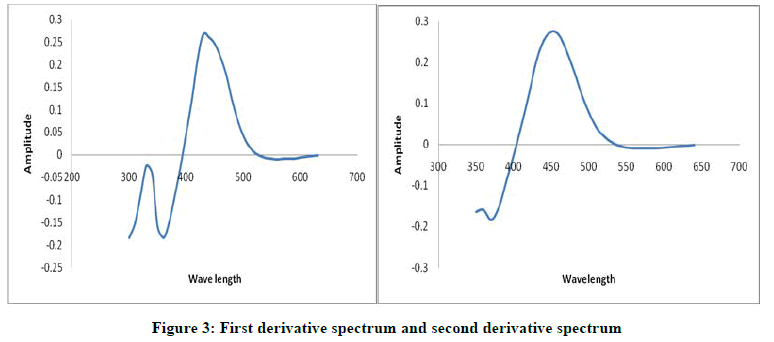
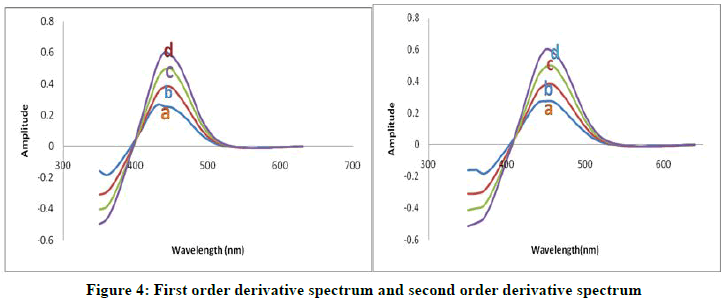
The first order and second order derivative spectral graphs shown in Figures 3 and 4 respectively. This shows that, the derivative amplitudes measured at λmax 435 nm for first order and 449 nm for second order were found to be proportional to the amount of Arsenic (III).
(As (III))=2.5 × 10-5 M
(DMBIH)=2.5 ×10-4 M
pH=8.5
SDBS (5%)=0.5 ml
As (III) (μg/ml)=A) 2.172; B) 3.158; C) 4.344; D) 5.380
(DMBIH)=5 × 10-4 M
pH=8.5
SDBS (5%)=0.5 ml
The stoichiometry of the complex was found to be 1: 1 (metal: ligand) investigated by Job’s continuous variation method and molar ratio method, with a stability constant 4.17 × 106. The effect of various diverse ions in the determination of of 1.87 μ g/mL As (III) was studied to find out the tolerance limit of foreign ions in the present method. The tolerance limit of a foreign ion was taken as the amount of foreign ion required to cause an error of ± 2% in the absorbance or amplitude. The results are given in Table 3. The data obtained in the derivative method is also incorporated. The data suggest that several associated anions and cations do not interfere when they are present in large excess, such as iodide, bromide, acetate, thiocyanide, tartarate, tetra borate, and chloride, strontium (i), manganese (II), barium (ii), zn (ii), bi (iii) and lanthanum (III). The tolerance limit values for many anions and cations are more in derivative method. The interference of associated metal ions such as iron (III) and Cu (II) are decreased by adding masking agents fluoride and thiourea (Table 4) [16-17].
| Ion added | Tolerance limit µg/ml | Ion added | Tolerance limit µg/ml |
|---|---|---|---|
| Bromide | 3296 | Bi+3 | 115 |
| Iodide | 2262 | Co2+ | 106 |
| Urea | 1575 | Zn2+ | 84 |
| Chloride | 1264 | Ca2+ | 76 |
| Tetraborate | 998 | Sn2+ | 73 |
| Sulphate | 965 | Zr4+ | 72 |
| Oxalate | 896 | Ni2+ | 62 |
| Nitrate | 635 | U6+ | 61 |
| Acetate | 590 | Ag+ | 55 |
| Thiocyanide | 613 | Mo6+ | 52 |
| Phosphate | 492 | Pb2+ | 2.1 |
| Ascorbic acid | 185 | Sb3+ | 47 |
| Tartarate | 166 | Cr3+ | 42 |
| Thiourea | 132 | Al3+ | 32 |
| Fluoride | 103 | Hg2+ | 10 |
| Ba2+ | 229 | Pd2+ | 12 |
| La3+ | 205 | Ru3+ | 6 |
| Sr2+ | 192 | Cu2+ | 2.12, 5.87* |
| Mn2+ | 122 | Fe3+ | 3.52, 8.72 ** |
Note: * Signifies masked with 615 μg/ml of thiourea; ** Signifies masked with 285 μg/ml of fluoride.
| Sample | Arsenic added μg/mL | Proposed method | AAS method | ||
|---|---|---|---|---|---|
| As +3 found in µg/mL ± SD | %Recovery | As +3 found in µg/mL ± SD | %Recovery | ||
| Serum | 2 | 1.98 ± 0.02 | 98.6 | 1.95 ± 0.05 | 98 |
| 4 | 3.86 ± 0.04 | 96.8 | 3.85 ± 0.03 | 96.3 | |
| 6 | 6.00 ± 0.07 | 100.1 | 5.98 ± 0.08 | 99.7 | |
| Urine | 2 | 1.98 ± 0.02 | 99 | 2.01 ± 0.04 | 99.8 |
| 4 | 3.95 ± 0.04 | 99 | 3.93 ± 0.06 | 98.3 | |
| 6 | 5.97 ± 0.02 | 99.5 | 5.94 ± 0.03 | 99 | |
| Hair | 2 | 1.98 ± 0.03 | 99 | 1.96 ± 0.03 | 98 |
| 3 | 3.05 ± 0.04 | 100.3 | 2.99 ± 0.03 | 99.7 | |
| 4 | 3.97 ± 0.02 | 99.5 | 3.99 ± 0.06 | 99.7 | |
| Nail | 2 | 1.98 ± 0.04 | 99 | 1.97 ± 0.06 | 98.5 |
| 3 | 2.95 ± 0.04 | 99.2 | 2.94 ± 0.02 | 98 | |
| 4 | 3.97 ± 0.03 | 99.5 | 4.01 ± 0.03 | 100.3 | |
Conclusion
Determination of As (III) in biological samples
Determination of arsenic in hair, serum, urine and nail: People drinking arsenic contaminated water have been reported to have high arsenic in their hair and nails. About 0.5-1.0 g of hair and nail sample were placed in a tubing containing nitric acid. The tube was closed and heated on hot plate at 100℃ for 5 min. After 24 h the lid was opened and 1 mL of nitric acid was added and evaporated at 100℃ until 1 mL solution remained. Few drops of 10% KI were added to convert As (V) to As (III). The presence of any excess of iodine, indicated by light brown colour was destroyed by adding few drops of ascorbic acid. The sample was cooled, diluted to 5 M and analyzed by the proposed and also by reported method. An aliquot (10-50 ml) of the sample solution was taken individually and arsenic was determined by the general procedure.
Acknowledgements
Authors thank to Jawaharlal Nehru Technological University Anantapur for providing research facilities to the research scholar.
References
- Chakraborti D, Mandal BK, Dhar RK, et al. Ind J Environ Prot. 1999, 19: 565.
- American Water Works Association, 1992.
- Kohlmeyer U, Jantzen E, Kuballa J, et al. Anal Bional Chem. 2003, 377(1): 6.
- Alvarez_Llamas G, de la Campa MR and Sanz Medel A. Anal Chim Acta. 2005, 546(2): 236.
- Sanz E, Munoz Olivas R, Camara C, et al. J Environ Sci Health. 2007, 42(12): 1695.
- Anezaki K, Nukatsuka I and Ohzeki K. Anal Sci. 1999, 15( 9): 829.
- Bundelaska JM, Stafilov T and Arpadjan S. Int J Environ Anal Chem. 2005, 85(3): 199.
- Gomez MM, Kovecs M, Palacios MA, et al. Microchim Acta. 2005, 150(1): 9.
- Ferreira MA and Barros AA. Anal Chim Acta. 2002, 459(1): 151.
- Kopanica M and Norotny L. Anal Chim Acta. 1998, 368(3): 211.
- Boadu M, Osae EK, Golow AA, et al. J Radioanal Nucl Chem. 2001, 249(3): 581.
- Dasgupta PK, Huang HL, Zhang GF, et al. Talanta. 2002, 58(1): 153.
- Gupta VK and Agarwal S. Talanta. 2005, 65(3): 730
- Nakai I, Baba Y, Tanaka K, et al. Chem Lett. 2008, 37(3): 304 (15).
- Taher MA. Analyst. 1995, 120: 1589.
- Patty FA. Ind Hyg Toxicol. 1963, 2: 871.
- Pillai A, Sunitha G and GuptaV K. Anal chim Acta. 2000, 408: 111.