Original Articles: 2022 Vol: 14 Issue: 8
Preparation, Diagnosis and Evaluation of Some New Synthetic Aromatic Derivatives with Their Antimicrobial Activity
Ammar Abdulghani Qasim Yahya*
Department of Dental Basic Sciences and Organic Chemistry, University of Mosul, Mosul, Iraq
- Corresponding Author:
- Ammar Abdulghani Qasim Yahya
Department of Dental Basic Sciences and Organic Chemistry, University of Mosul, Mosul,
Iraq
Received: 25-Mar-2022, Manuscript No. JOCPR-22-58416; Editor assigned: 28-Mar-2022, PreQC No. JOCPR-22-58416 (PQ); Reviewed: 11-Apr-2022, QC No. JOCPR-22-58416; Revised: 24-May-2022, Manuscript No.JOCPR-22-58416 (R); Published: 31-May-2022 DOI: 10.37532/0975-7384.2022.14(8).1-11
Abstract
The compound 2-aminobenzenethiol was reacted with malononitrile at RT to produce 2-(benzothiazol-2-yl) acetonitrile (H1), it was reacted with different carbonyl compounds to get derivatives of 2-(benzothiazol-2-yl)-3-(2-hydroxy-4-methoxyphenyl) acrylonitrile (H2-H10). It was also treated with sodium azide in the existence of the ZnCl2 to produce (H11-H14) on a side, while it was reacted some of the benzothiazole derivatives with sodium azide without a catalyst to produce (H15-H17) on the other side. The chemical compositions of the prepared compounds were diagnosed and confirmed by spectroscopies of UV, FT-IR and 1H-NMR. The effect of these compounds on some bacteria and a fungus was investigated, good results were obtained consequently.
Keywords
Benzothiazole; Triazole; Imidazotriazine
Introduction
The chemistry of heterocyclic is very important in everyday use; it has a wide range of applications in (Medicine and industrial) compounds. The benzothiazoles represent the basic structure of the various prepared compounds that have a wide spectrum of biological activities such as anti-cancer effects, necrosis inhibitors and fatty acids[1]. Some isoxazoles and pyrazolates have antimicrobial activities [2]. The therapeutic properties of heterocyclic compounds have also encouraged researchers in the field of clinical chemistry to preparmore derivatives [3]. Benzothiazole has a system of particular interest in the field of medicinal chemistry due to its remarkable pharmacological potential including anticancer, antimicrobial, anti-diabetic, etc. [4]. The misuse of antibiotics since the discovery of penicillin has recently led to the urgent need for new antibiotics, due to the emergence of bacterial strains that have great resistance to antibiotics; therefore, the use of plant extracts or pure natural compounds with conventional antibiotics is successful subject [5]. The applications of benzothiazole is a wide range of use, due to its limitless pharmacological effects, 2-aminobenzothiazole. It allows the combination with heterocyclic compounds to achieve a new pharmacological effect and reduce toxicity [6]. Benzotriazoles with triazole exhibit wide potentialities in medicine, since some of them have been shown to inhibit the growth of cancerous cells [7]. Infections affecting the digestive system may be the cause of disease and death. Medicines for nitrogenous cyclic compounds have expanded their applications and newly-used to treat anaerobic bacteria. There is a great interest in reducing nitroimidazole to produce highly effective drugs, in addition to their relationships between the formulation and mutagenesis [8]. The heterocyclic compounds are the basic and important units in medicinal chemistry; it contains the elements sulfur, oxygen and nitrogen, for example, thiophene, thiazole, furan, imidazotriazine, diazeridine, etc. Features of these reactions depend on the structure of reactants, intermediates and reaction medium (organic solvents, ionic liquids) [9].
Materials and Methods
The chemical reagents used in the preparation were purchased from Fluka and PDH. Melting points were measured using the device, which has not been corrected. The infrared spectra were measured by using ATR-Diamond. 1H-NMR spectra were measured by using a (Brucker 400 MHz) spectrometer using CDCl3/DMSO-d [6].
Experimental
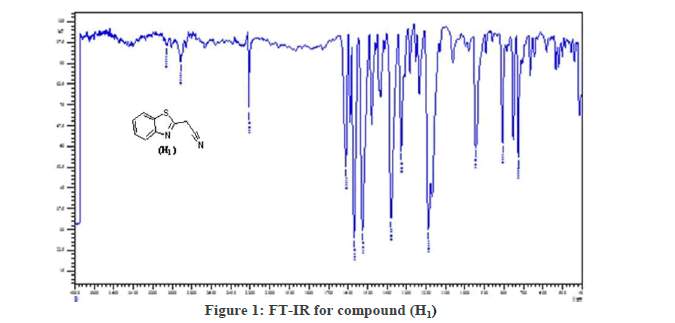
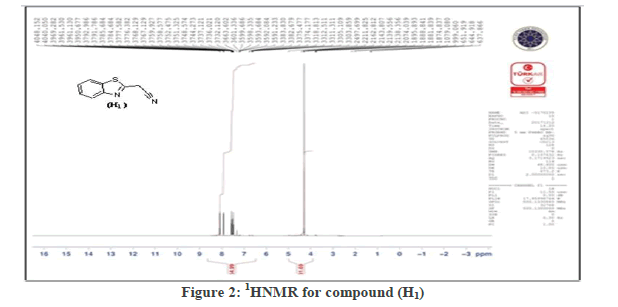
Synthesis of 2-(benzothiazol-2-yl)acetonitrile (H1):(S1): The compound 2-aminobenzenethiol (5 g, 0.04 mol and malononitrile (2.64 g ,0.04 mol)) was dissolved in (30 ml) Absolute ethanol, (6 ml) of glacial acetic acid were added. Stirrer the mixture overnight at RT, filter and recrystallized with ethanol to get A Yellow precipitate has m.p.(100-101°C) with (85%). The FT-IR spectrum showed spectrum bands at (3055 cm-1 str. C-H Ar.), (2925 C-H alf. str.; 2200 cm-1 CN str.; 1652 cm-1 C=N str.; 1558 cm-1 , 1431 cm-1 C=C Ar. str.; 756 cm-1;C-S-C str. 1H-NMR spectrum showed signals between 7.15-8.1 ppm for four aromatic hydrogen and the signal at 4.3 ppm due to (2H, s).
(S2): General synthesis method of 2-(benzothiazol-2-yl)-3-phenylacrylonitrile derivatives [11-15] (H2-H10), (0.0015 moles) of the (H1) was dissolved in absolute ethanol (Table 1). 0.0015 moles of aromatic (aldehydes or ketones) were added to the mixture; (8-10) drops of piperidine were added. The mixture stirred for six hrs, at (RT), filters and recrystallized with ethanol (Figures 1 and 2).
| Comp. | R | M.P. (ºC) | Color | Yield (%) |
|---|---|---|---|---|
| H2 | 4-NMe2 | 234-236 | Deep red | 95 |
| H3 | 4-Br | 104-105 | Red | 89 |
| H4 | 4-Cl | 152-153 | Deep green | 92 |
| H5 | 4-OMe-2-OH | 178-178 | Orange | 82 |
| H6 | 2-NO2-2-OH | 190-192 | Hazel | 79 |
| H7 | 4-OH | 101-102 | Yellow | 94 |
| H8 | 2-OH | 184-185 | Yellow | 98 |
| H9 | H | 88-89 | Pale Yellow | 93 |
| H10 | 4-OMe | 144-146 | Deep Yellow | 97 |
| H11 | 4-OMe | 300-301 | Brown | 75 |
| H12 | 4-NMe2 | 209-210 | Red | 67 |
| H13 | 4-Cl | 44-46 | Green | 53 |
| H14 | 2-OH | 214-216 | Red | 59 |
| H15 | 4-OMe | 135-137 | Red | 54 |
| H16 | 4-Cl | 114-116 | Red | 66 |
| H17 | 2-OH | 296-299 | Red | 48 |
Table 1: Physical properties of the synthesis compounds
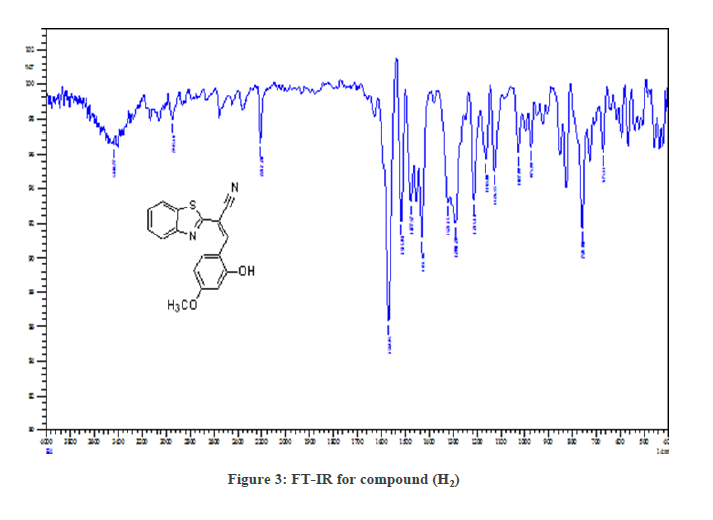
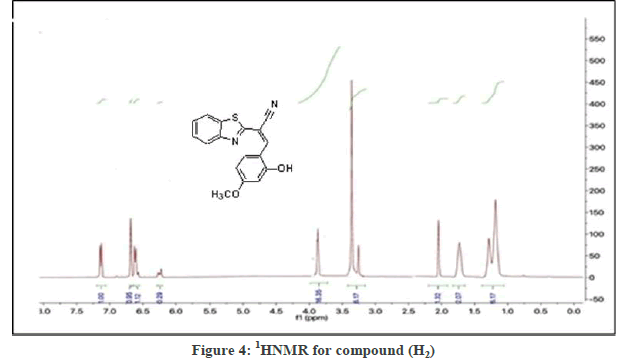
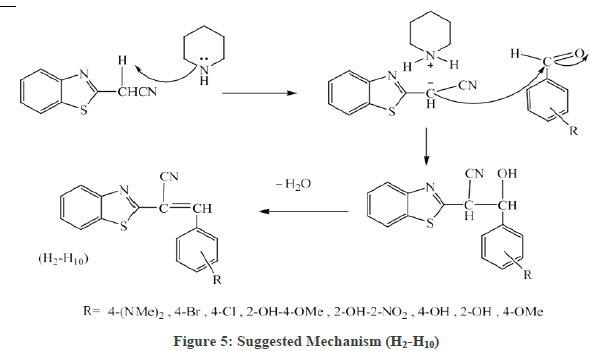
Figure 3 the FT-IR spectrum showed the bands of the (H2) compounds at (1612-1697cm-1str. C=H), for all of the synthesized compounds, it proofs the formation the compounds bands, other bands listed in Table 2. In the Figure 4, 1H-NMR spectrum of (H2) compound showed signals between 7.15-8.45 ppm for four aromatic hydrogen, a signal at 5.19 ppm, signal at 1.63 ppm (1H, s ) was attributed to (-CH) and a signal at 11 due to (OH,s) as shown in Table 2. The suggested mechanism for this reaction illustrated in the Figure 5.
| Comp. No. |
I.R., υ (cm-1), KBr | 1H-NMR υ (ppm), CDCl3 |
|||
|---|---|---|---|---|---|
| OH | C=C | C=N | -C=C Ar. | ||
| H2 | - | 1612 | 1568 | 1477 | |
| H3 | - | 1610 | 1589 | 1431 | |
| H4 | 1649 | 1558 | 1492 | 6.8 - 8.13 (m, 4H, Ar-H); 1.09 (s,CH) | |
| H5 | 3440 | 1650 | 1569 | 1515 | |
| H6 | 3477 | 1697 | 1610 | 1610 | |
| H7 | 3276 | 1625 | 1590 | 1514 | |
| H8 | 3236 | 1662 | 1604 | 1458 | 6.8 - 8.13 (m, 4H, Ar-H); 1.09- 3.31 (S,2H, NHNH); 4.35 (d, 2H, CH2); 10 (S,1H,OH) |
| H9 | - | 1639 | 1600 | 1448 | |
| H10 | - | 1600 | 1591 | 1512 | 6.8 - 8.13 (m, 4H, Ar-H); 1.09- 3.31 (s,CH) 4.35 (s, 3H, OH3); |
Table 2: Spectral data of compounds (H2- H10)
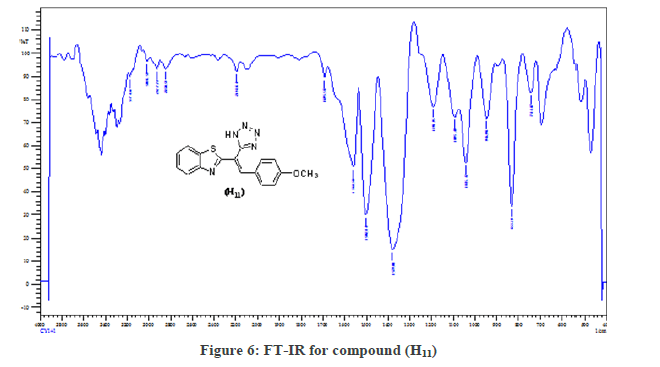
(S3a): General Synthesis method of Tetrazoles (H11-H14): (0.002 mol) of the compounds (H2-H10), sodium azide (0.003 mol), and (0.003 mol) of ZnCl2 were dissolved in (25 ml) DMF. The reaction was refluxed for 10 hrs. The mixture diluted with water, Filter and recrystallized with DMF. In the Figure 6 the FT-IR spectrum showed disappear the CN group at 2200 cm-1 and appeared the N3 group at (2400 cm-1) and the N=N group at (998 cm-1). However, while proccing the reaction without a catalyst use the (-C=C-) group disappeared at (1600-1658 cm-1) those are good evidence on the success the reaction, other FT-IR bands listed in Table 3.
| Comp. No. |
I.R., υ (cm-1), KBr | 1H-NMR υ (ppm), CDCl3 |
|||
|---|---|---|---|---|---|
| NH | C-H alf. | C=C | N=N | ||
| H11 | 3267 | 2927 | 1693 | 946 | |
| H12 | 3060 | 2923 | 1652 | 937 | |
| H13 | 3127 | 2895 | 1659 | 998 | |
| H14 | 3051 | 2923 | 1652 | 956 | 6.8 - 8.13 (m, 4H, Ar-H); 1.09 |
| (S,1H, NH); 4.35 (s, 1H, CH); | |||||
Table 3: Spectral data of compounds (H11-H14)
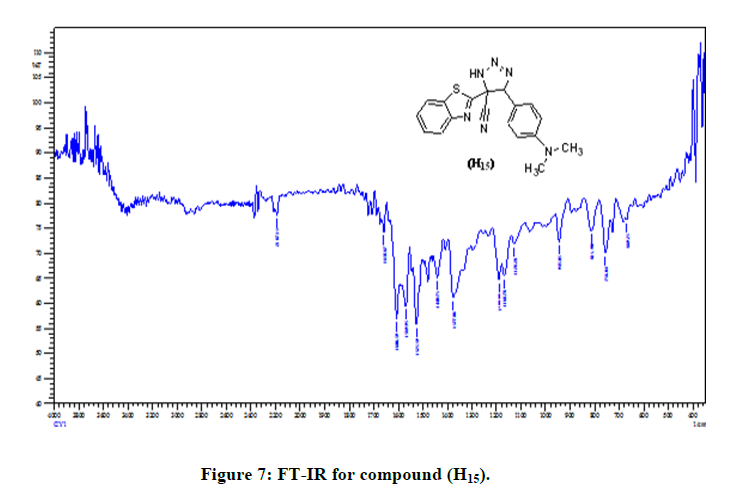
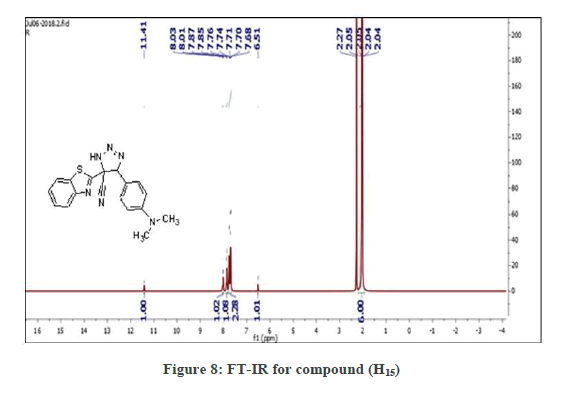
(S3b): General synthesis method of Triazoles (H15-H17): 0.002 moles of the (H2-H10), compounds, and (0.003 mol) sodium azide was dissolved in (20 ml) DMF. The mixture was refluxed for 24 hrs. Diluted with Water, acidify until the precipitate formed with conc. HCl, Filter and recrystallized with DMF. In this reaction, the cyclation occurred on (-C=C-) instead of occurring on nitrile group. In the Figure 7 the FT-IR spectrum showed bands at (2187 cm-1) due to (N3) group, (3055 cm-1 C-H Ar. str.; 2200 cm-1 CN str. good evidence on the success the reaction, that are listed in Table 4. 1H-NMR spectrum of compound (H15), showed signals between 7.68-8.03 ppm for aromatic hydrogen, a signal at 2-2.3 ppm due to (N(CH3)2, s), a signal at 6.51 ppm due to (CH, s) and a signal at 11.41 ppm due to (NH , s) (Figure 8).
| Comp No. | I.R., υ (cm-1), KBr | 1H-NMR, υ (ppm), d6-MSO | |||
|---|---|---|---|---|---|
| NH | C-H alf. | C=N | N=N | ||
| H15 | 3265 | 2900 | 2187 | 945 | 2.0-2.3(N(CH3)2,6.51CH ,7.68 -8.03 Ar-CH,11.41 NH |
| H16 | 3298 | 2925 | 2059 | 952 | 6.58CH ,7.57 -7.89 Ar-CH ,11.24 NH |
| H17 | 3200 | 2900 | 2360 | 833 | 3.5 (OCH3) ,6.69 CH ,7.78 -8.08 Ar-CH,11.45 NH |
Table 4: Spectral data of compounds (H15–H17)
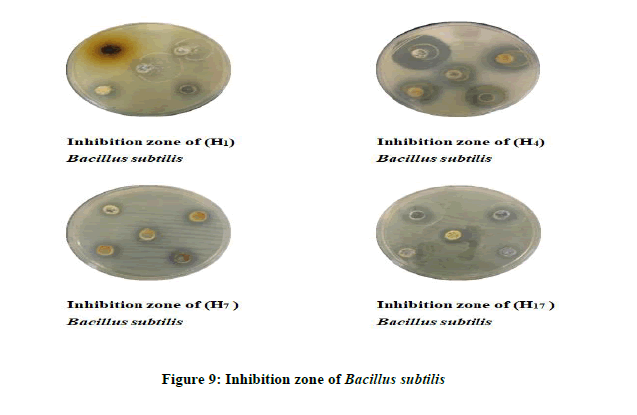
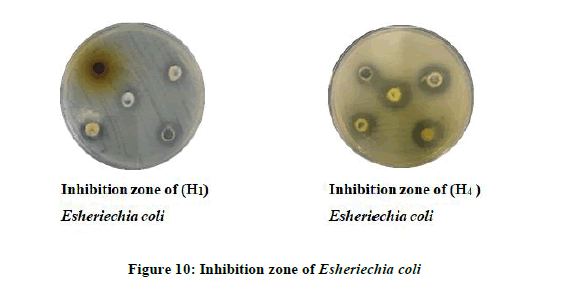
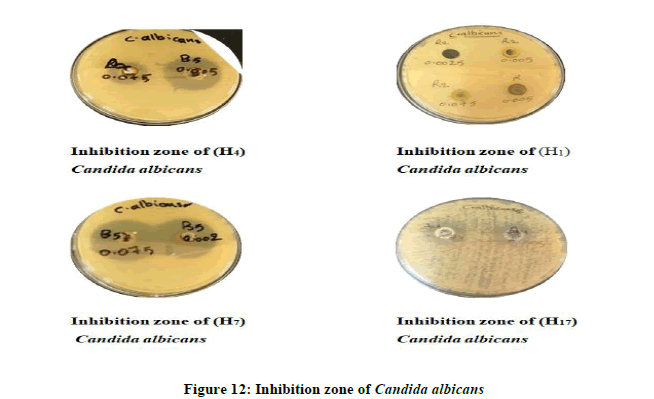
Antimicrobial Activity of some of the synthesized compounds: A preliminary evaluation of antibacterial and anti-fungus activity against types of bacteria like Escherichia coli, Bacillus subtilis (Gram-negative) and Candida albicans fungus. Those kinds of bacteria and (Figure 9) fungus have been choosing because of their wide importance in the clinical field, it causes many diseases their various resistances of the antibiotic and chemical drugs. All of the tested compounds were studied at different concentrations by using DMSO as a solvent (0.05, 0.001, 0.075, 0.005, and 0.0025 mg/ml) (Figure 10 and Table 4).
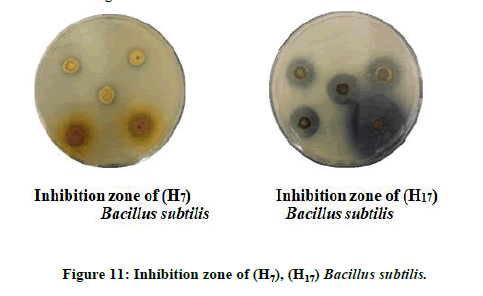
The results showed that the most of the tested compounds have good antibacterial and antifungal activity since they have active groups in their molecules, as well as choosing different concentrations of these compounds. The inhibition zone is from (16 mm the lowest inhibition zone to 36 mm the highest inhibition zone of Fungus) (Figures 11 and 12). As for bacteria, it is about (10 mm the lowest inhibition zone to 32 mm the highest inhibition zone of Bacteria). According to the antibacterial studies, the efficacy of the compounds against Gram-positive bacteria is higher than Gram-negative bacteria [16]. The activities were illustrated in (Table 5).
| Comp. | (zone of inhibition in mm) | (zone of inhibition in mm) | ||
|---|---|---|---|---|
| Conc. | Gram. (-) Bacteria Escherichia coli g/mlμ |
Gram. (+) bacteria Bacillus | Candida albicans | |
| H1 | 0.005 | 16 | 25 | 20 |
| 0.07 | 17 | 19 | 17 | |
| 0.025 | 18 | 20 | 25 | |
| H4 | 0.01 | 16 | 20 | 36 |
| 0.005 | 15 | 17 | 35 | |
| 0.15 | 25 | 31 | - | |
| H7 | 0.01 | - | - | 17 |
| 0.005 | - | - | - | |
| 0.15 | 16 | 25 | 27 | |
| H17 | 0.005 | 17 | 19 | - |
| 0.0025 | 25 | - | ||
| 0.075 | 19 | - | ||
| Ampicillin | 20 | 20 | - | |
| Streptomycin | 25 | |||
Table 5: The antimicrobial activities
Conclusion
Hydrazones have become one of the most important heterocyclic in current chemistry research, due to its important pharmaceutical applications, especially in biological science, and medicinal chemistry. New derivatives of benzothiazole rings were synthesized in this research included a new methodology for a synthesis of hydrazones. All the synthesis derivatives were identified characteristic by some physical properties and surly analyzed by FTIR and1HNMR. All the derivatives exhibited good varied antimicrobial activity.
References
- Havrylyuk D, Mosula L, Zimenkovsky B, et al. Eur J Med Chem. 2010;45:5012-5021.
- Hamada NM, Sharshira EM. Molecules. 2011; 16(3):2304-2312.
- Crossref
- Ali R, Siddiqui N. J Chem. 2013; 345198.
- Cheesman MJ, Ilanko A, Blonk B, et al. Pharmacogn Rev. 2017;11(22):57–72.
- Singh P, Srivastava A, Mishra AP, et al. J Drug Discov Dev. 2017; 1(1):4-12.
- Korcz M, Saczewski F, Bednarski PJ, et al. Molecules. 2018;23(6):1497.
- Olender D, Zwawiak J, Zaprutko L, et al. Pharmaceuticals (Basel). 2018;11(2):54.
- Makhova NN, Belen’kii LI, Gazieva GA, et al. Russ Chem Rev. 2020;89(1):55.
- Bhagat S, Sharma N, Chundawat TS, et al. J Chem. 2013;2013.
- Maddila S, Jonnalagadda SB, et al. Bull Chem Soc Ethiop. 2012; 26(3):467-471.
- Habibi D, Nasrollahzadeh M. Monatsh fur Chem. 2012; 143(6):925-930.
- Nasrollahzadeh M, Sajjadi M, Tahsili MR, et al. Acs Omega. 2019;4(5):8985-9000.
- Parminder Kaur, Rajwant Kaur, Manish Goswami. Int Res J Pharm. 2018;9:(7).
- Gabriel O, Resende SF, Alexandre A, et al. Int J Electrochem. 2019;6759478.
- Halim NI, Kassim K, Fadzil AH, et al. APCBEE Procedia. 2012;3:129-133.