Original Articles: 2023 Vol: 15 Issue: 2
Phytochemical Screening and Antibacterial Efficacy of Pinda concanensis (Dalzell) PK Mukh and Constance
Komal Shivaji Walvekar, Mansingraj Shahajirao Nimbalkar*
Department of Botany, Shivaji University, Kolhapur, Maharashtra, India
Received: 11-Oct-2022, Manuscript No. JOCPR-22-77439; Editor assigned: 13-Oct-2022, PreQC No. JOCPR-22-77439 (PQ); Reviewed: 27-Oct-2022, QC No. JOCPR-22-77439; Revised: 09-Jan-2023, Manuscript No. JOCPR-22-77439 (R); Published: 16-Jan-2023
Abstract
Pinda concanensis (Dalzell) PK Mukh and Constance is belongs to family Apiaceae and is commonly known as Konkan Pinda. This is the first report on phytochemical analysis and antimicrobial potential of the aqueous and methanolic extracts of leaves, roots and stem has been evaluated against important bacterial strains gram negative Escherichia coli and gram positive Bacillus cereus. This aqueous and methanolic extract of different plant parts of the Pinda concanensis was subjected to determine total content of the phytochemicals like alkaloids, saponins and terpenoids. Methanolic extract of dry root exhibit the highest content of total alkaloid (1.4706 ± 0.0156 mg CE/g DW) and the highest content of total terpenoids (2.3632 ± 0.040 mg UAE/g DW). While methanolic extract of dry stem showed the highest content of total phenolic (0.900 ± 0.023 mg TAE/g DW). The antibacterial activity was determined by using the micro dilution method by calculating MIC of all the plant parts examined, it was discovered that the methanolic extracts were more powerful than the aqueous extracts. The result demonstrates that the methanolic extract of different parts of Pinda concanensis, especially the root could be developed as pharmaceutical products.
Keywords
Pinda concanensis; Phytochemicals; Antibacterial activity; Gram-positive bacteria; Gram-negative bacteria
Introduction
Phytochemicals are the chemicals produces by various parts of the plants. These bioactive constituents of plants are phenolics, flavonoids, steroids, terpenoids, carotenoids, alkaloids, tannins and glycosides. These compounds have various activities such as antibacterial some have been reported to exhibit heamolytic and foaming activity reported [1]. According to Fransworth and Morris, the majority of natural products are secondary metabolites, of which unevenly 12,000 have been identified thus far [2]. Plants use these products to protect themselves against microbes, insects and herbivores. The use of crude extracts and dry powder samples from medicinal and aromatic plants for the creation and manufacturing of alternative traditional medicines and food additives is becoming increasingly popular. For the sake of public health, it is crucial to utilize preservatives and antibacterial substances to restrict the growth of dangerous microorganisms in food [3]. Plants from family Apiaceae are commonly used for flavoring of foods and in medicine. The plant under present investigation Pinda concanensis (Dalzell) belonging to family Apiaceae is a medicinal plant from Western Ghats of India. The synonym of Pinda is Heracleum pinda (Dalzell and Gibson). It is an annual herb with tuberous roots, which are eaten raw by local folklore. This plant is heavily exploited for its medicinal uses, especially for the essential oil extracted from its seeds [4]. The plant Pinda concanensis has the potential of antioxidant, antimicrobial activity. There are very few studies on phytochemical investigation of Pinda concanensis. The current work used distilled water and methanol as solvents to extract secondary metabolites such as phenolic, flavonoid, saponins, alkaloid and terpenoids from Pinda concanensis and its antibacterial activity against gram positive and gram negative bacteria like Bacillus cereus and Escherichia coli respectively and quantitative analysis [5].
MATERIALS AND METHODS
Collection of plant material
The plant Pinda concanensis were collected from Kas plateau, Satara latitude-17°43′12.58″N to 17°72'01.61"N and longitude 73°49′ 22.05″E to73°82' 27.92"E. The plants were identified, germplasm of these plants were maintained in the lead botanical garden, department of botany, Shivaji University, Kolhapur. A voucher specimen (KSW 001) was deposited in the herbarium of department of botany, Shivaji University, Kolhapur [6].
Preparation of extracts
The fresh and dry plant material extract was prepared by using two solvents; methanol and distilled water. The different parts of plant (25 g) were ground using laboratory grinder. The extracts were then filtered through Whatman filter paper no.1 by using Buchner’s funnel and final volume was adjusted to100 ml with respective solvents. For each plant part same extraction procedure with these two solvents was adopted [7]. All the plant extracts (25%) were stored in refrigerator at 4°C and were used for further analysis.
Analysis of total phenolic
Total Phenolic Contents (TPC) of the plant extracts were assessed using Folin-Ciocalteu method with slight modifications. The reaction mixture was prepared by mixing an aliquot of extracts (12.5 μl) with distilled water (0 μl) and Folin-Ciocalteu reagent (12.5 μl) incubate 10 minutes and 125 μl of saturated Na2CO3 solution. Reaction mixture was further incubated for 90 minutes at room temperature in dark [8]. The absorbance was measured at 760 nm in 96 well microplate. The assays were prepared in triplicates for each analysis and the mean values of absorbance were obtained [9]. The readings were compared with a standard phenolic compound i.e. tannic acid and were expressed as milligram Tannic Acid Equivalents per gram Fresh Weight/Dry weight (mg TAE of DW or FW) [10].
Analysis of total flavonoids
Total Flavonoids Content (TFC) of the different plant parts were analyzed by using modified colorimetric method. The reaction mixture was prepared by mixing 150 μl 2% methanolic AlCl3 with 150 μl plant extract. Incubation of 10 minutes at room temperature was done. After incubation the absorbance was measured at 368 nm in 96 well microplates [11]. The assays were prepared in triplicates for each analysis and the mean values of absorbance were obtained. The values were expressed as milligram Quercetin equivalents per gram Fresh Weigh/Dry Weight (mg QE/g of DW or FW) [12].
Quantification of total saponin
Saponin content in Pinda concanensis was determined according to the method described with slight modification. Total Saponins also known as vanillin-sulfuric acid assay. In this, reaction mixture consists of 50 μl of plant extract, 50 μl of 8% vanillin to which 500 μl of 72% H2SO4 was added. Reaction mixture was incubated for 10 min at 60°C in water bath [13]. Allow cooling and absorbance was measured at 544 nm (Multiskan sky spectrophotometer, thermo scientific) wavelength. The total saponin content was calculated from the calibration curve and the results were expressed as mg of diosgenin equivalent per gm fresh or dry weight [14].
Quantification of total alkaloids
Total Alkaloid Content (TAC) of the extracts was assessed using 1, 10-phenanthroline method described by Singh. The assay mixture was prepared by using 100 μl plant extract 100 μl 1,10-phenanthroline and 100 μl FeCl3 in 0.5 M HCl, make a total volume 1 ml by using distilled water and incubated for 30 minutes in water bath maintained at 70 ± 2°C (Till orange color appear) [15]. Above reaction mixture excluding 1% plant extract, substituted by distilled water served as a blank. The samples were prepared in triplicates for each analysis and the mean value of absorbance was obtained. The absorbance was measured at 510 nm in 96 well microplate against reagent blank. (Multiskan sky spectrophotometer, thermo scientific). The OD measurements were compared to standard curve of colchicines (a standard alkaloid) and expressed as milligrams Colchicine Equivalent (CE) per gram of Fresh Weight (FW) or Dry Weight (DW) of respective plant parts of Pinda concanensis [16].
Quantification of total terpenoid
Total triterpenoid content was determined by colorimetry using the following procedure, 25 μl of plant extract was mixed with 37.5 μl vanillin-glacial acetic acid solution (5% w/v) and 125 μl of perchloric acid [17]. Then samples were heated for 45 min at 60°C and cooled on ice bath. Absorbance was measured at 548 nm wavelength after the addition of 562.5 μl of glacial acetic acid, using 96 well plate reader (Multiskan sky spectrophotometer, thermo scientific). The total terpenoid content was calculated from the calibration curve of ursolic acid and the results were expressed as mg of ursolic acid equivalent per gm fresh or dry weight [18].
Antibacterial activity
Preparation of nutrient broth: The nutrient broth was prepared by using nutrient powder without agar containing only nutrient ingredients are peptone (5 gm./liter), sodium chloride (5 gm/liter), HM peptone B#(1.50 gm./liter), yeast extract (1.50 gm./liter) (# equivalent to beef extract) final PH (25°C) 7.4 ± 0.2. For bacterial growth 13 grams dissolved in 1000 ml distilled water. Heat if necessary to dissolve the medium completely [19]. Dispense into tubes or flasks as desired. Sterilized by autoclaving at 15 lbs pressure (121°C) for 15 minutes.
Bacterial culture: Escherichia coli, (Gram -ve) Bacillus cereus (Gram +ve). Strains were collected from National Center for Microbial Resource (NCMR); all bacterial strains were retrieved for fresh culture in nutrient broth [20]. For growth curve study each freshly grown strain was inoculated in to nutrient broth and incubated for 12 hours at 37°C. Absorbance was recorded after every 30 min.
Anti-microbial activity: The antimicrobial activity of Pinda concanensis was carried out by the following method. In 96 well plate 200 μl nutrient broth was distributed into each well, 25 μl of plant samples with different concentrations (1%, 2%, 3%, 4% and 5%) to determine MIC and 20 μl bacterial culture was supplemented to each well [21]. Plate was incubated at 37°C for 10 hours in a spectrophotometer (Multiskan sky 96 well plate reader, thermo scientific). Absorbance was recorded at 600 nm. After incubation, the growth curve was plotted for the analysis of plant samples inhibitory activity [22]. Gentamicin (20 μg/ml to 100 μg/ml) was used as standard positive control while methanol and water as negative control. Percentage of inhibition was calculated by,
Percentage of inhibition (%)={(A0–A1)/A0} × 100
Whereas,
A0 =Absorbance of bacterial growth.
A1=Absorbance of sample inhibiting bacterial growth.
RESULTS
Total Phenolic Content (TPC)
The total phenolics are depicted in Table 1 and Figure 1. The highest amount of Total Phenolic Content (TPC) was observed in methanolic extract of dry stem (0.900 ± 0.023 mg TAE/g of DW) and lowest value was observed in methanolic extract of fresh stem (0.302 ± 0.002 mg TAE/g of FW) [23]. The dry root showed highest value of Total Phenolic Content (TPC) in aqueous extract (0.194 ± 0.005 mg TAE/g of DW) and lower amount of total phenolic content was observed in aqueous extract of fresh root (0.085 ± 0.001 mg TAE/g of FW) [24].
| Solvent | Plant sample | Total phenolic content* ± SE | |
|---|---|---|---|
| Methanol | Fresh | Root | 0.131 ± 0.002 |
| Stem | 0.302 ± 0.002 | ||
| Leaf | 0.263 ± 0.002 | ||
| Dry | Root | 0.137 ± 0.005 | |
| Stem | 0.900 ± 0.023 | ||
| Leaf | 0.421 ± 0.001 | ||
| Aqueous | Fresh | Root | 0.085 ± 0.001 |
| Stem | 0.489 ± 0.001 | ||
| Leaf | 0.186 ± 0.005 | ||
| Dry | Root | 0.194 ± 0.005 | |
| Stem | 0.786 ± 0.037 | ||
| Leaf | 0.214 ± 0.001 | ||
| Note: Values in the table are expressed as mean ± SD of triplicate measurements. *mg TAE/g FW or DW: Milligram tannic acid equivalents per gram fresh weight or dry weight. | |||
Table 1: Total phenolic content in Pinda concanensis.
Total Flavonoids Content (TFC)
The highest value of total flavonoid content was observed in aqueous extract of dry stem (0.038 ± 0.001 mg QE/g of DW) and methanolic extract of dry stem show lowest value (0.011 ± 0.004 mg QE/g of DW) which are recorded in Table 2 and Figure 2. The methanolic dried root extract exhibited highest flavonoid value (0.0093 ± 0.003 mg QE/g of DW) followed by methanol extract of fresh root (0.0057 ± 0.006976 mg QE/g of FW) [25].
| Solvent | Plant Sample | Total flavonoid content** ± SE | |
|---|---|---|---|
| Methanol | Fresh | Root | 0.0056 ± 0.007 |
| Stem | 0.018 ± 0.005 | ||
| Leaf | 0.0040 ± 0.001 | ||
| Dry | Root | 0.0093 ± 0.003 | |
| Stem | 0.011 ± 0.004 | ||
| Leaf | 0.019 ± 0.006 | ||
| Aqueous | Fresh | Root | 0.0064 ± 0.003 |
| Stem | 0.021 ± 0.004 | ||
| Leaf | 0.016 ± 0.004 | ||
| Dry | Root | 0.009 ± 0.002 | |
| Stem | 0.038 ± 0.001 | ||
| Leaf | 0.036 ± 0.001 | ||
| Note: Values in the table are expressed as mean ± SD of triplicate measurements. **mg QE/g FW or DW: Milligram quercetin equivalents per gram fresh weight or dry weight. | |||
Table 2: Total flavonoid content in Pinda concanensis.
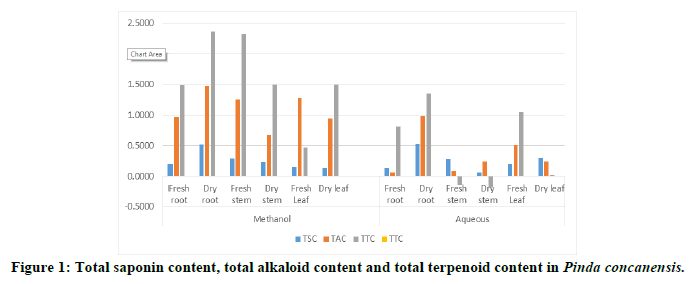
Total Saponin Content (TSC)
The highest value of saponin content was observed in aqueous extract of dry root (0.5286 ± 0.011 mg DE/g of DW) which was followed by methanolic extract of dry root (0.5211 ± 0.019 mg DE/g of DW). The lowest value of saponin content was found in aqueous extract of dry stem (0.0612 ± 0.008 mg DE/g of DW) which were recorded and observed in Table 3 [26]. But dry leaf of methanolic extract and fresh root of aqueous extract showed same value of saponin content (0.1374 ± 0.006 mg DE/g of FW or DW).
| Solvent | Plant sample | Total saponin content*** ± SE | |
|---|---|---|---|
| Methanol | Fresh | Root | 0.1936 ± 0.054 |
| Stem | 0.2935 ± 0.002 | ||
| Leaf | 0.1462 ± 0.006 | ||
| Dry | Root | 0.5211 ± 0.019 | |
| Stem | 0.2318 ± 0.003 | ||
| Leaf | 0.1374 ± 0.006 | ||
| Aqueous | Fresh | Root | 0.1374 ± 0.006 |
| Stem | 0.2809 ± 0.001 | ||
| Leaf | 0.1915 ± 0.011 | ||
| Dry | Root | 0.5286 ± 0.011 | |
| Stem | 0.0612 ± 0.008 | ||
| Leaf | 0.3030 ± 0.005 | ||
| Note: Values in the table are expressed as mean ± SE of triplicate measurements. ***mg DE/g FW or DW: Milligram Diosgenin equivalents per gram fresh weight or dry weight. | |||
Table 3: Total saponin content in Pinda concanensis.
Total Alkaloid Content (TAC)
The highest value of total alkaloid content was found in dry root of methanolic extract (1.4706 ± 0.0156 mg CE/g of DW) and lowest value of total alkaloid content was observed in fresh root of aqueous extract (0.0600 ± 0.003 mg CE/g of FW). Overall the total alkaloid content found more in methanolic fresh and dry extracts than aqueous extracts of Pinda concanensis which was described in Table 4 [27].
| Solvent | Plant sample | Total alkaloid content**** ± SE | |
|---|---|---|---|
| Methanol | Fresh | Root | 0.9717 ± 0.0095 |
| Stem | 1.2572 ± 0.0268 | ||
| Leaf | 1.2706 ± 0.0027 | ||
| Dry | Root | 1.4706 ± 0.0156 | |
| Stem | 0.6761 ± 0.0037 | ||
| Leaf | 0.9406 ± 0.0041 | ||
| Aqueous | Fresh | Root | 0.0600 ± 0.003 |
| Stem | 0.0846 ± 0.006 | ||
| Leaf | 0.5086 ± 0.004 | ||
| Dry | Root | 0.9850 ± 0.021 | |
| Stem | 0.2433 ± 0.010 | ||
| Leaf | 0.2407 ± 0.004 | ||
| Note: Values in the table are expressed as mean ± SE of triplicate measurements. ****mg CE/g FW or DW: Milligram colchicine equivalents per gram fresh weight or dry weight. | |||
Table 4: Total alkaloid content in Pinda concanensis.
Total Terpenoid Content (TTC)
The total terpenoid content of plant parts was depicted in Table 5. The highest total terpenoid content was found in methanolic extract of dry root (2.3632 ± 0.040 mg UAE/g of DW).while the lowest value of total terpenoid content was observed in aqueous extract of dry leaf (0.0015 ± 0.003 mg UAE/g of DW).The methanolic extract of fresh stem showed higher total terpenoid content (2.3248 ± 0.036 mg UAE/g of FW).
| Solvent | Plant Sample | Total Terpenoid Content***** ± SE | |
|---|---|---|---|
| Methanol | Fresh | Root | 1.4948 ± 0.049 |
| Stem | 2.3248 ± 0.036 | ||
| Leaf | 0.4698 ± 0.023 | ||
| Dry | Root | 2.3632 ± 0.040 | |
| Stem | 1.5032 ± 0.008 | ||
| Leaf | 1.5032 ± 0.035 | ||
| Aqueous | Fresh | Root | 0.8065 ± 0.019 |
| Stem | -0.1418 ± 0.001 | ||
| Leaf | 1.0482 ± 0.019 | ||
| Dry | Root | 1.3465 ± 0.025 | |
| Stem | -0.1852 ± 0.003 | ||
| Leaf | 0.0015 ± 0.003 | ||
| Note: Values in the table are expressed as mean ± SE of triplicate measurements. *****mg UAE/g FW or DW: Milligram ursolic acid equivalents per gram fresh weight or dry weight. | |||
Table 5: Total terpenoid content in Pinda concanensis.
Antibacterial activity
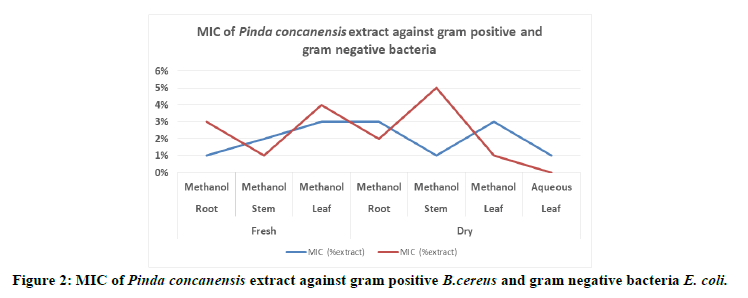
To compare the sensitivity of the bacterial strains to the studied extracts. Minimum Inhibitory Concentration (MIC) were determined and the results were illustrated in Table 6.The all methanolic extract of root, stem and leaf is highly effective at preventing the growth of these bacteria at a minimum concentration of extract. But aqueous extract of these plant parts did not showed any effect against these bacteria except aqueous extract of dry leaf.
| Plant part | Solvent | MIC (%extract) | ||
|---|---|---|---|---|
| Escherichia coli | Bacillus cereus | |||
| Fresh | Root | Methanol | 1% | 3% |
| Stem | Methanol | 2% | 1% | |
| Leaf | Methanol | 3% | 4% | |
| Dry | Root | Methanol | 3% | 2% |
| Stem | Methanol | 1% | 5% | |
| Leaf | Methanol | 3% | 1% | |
| Fresh | Root | Aqueous | Show poor activity | Show poor activity |
| Stem | Aqueous | |||
| Leaf | Aqueous | |||
| Dry | Root | Aqueous | ||
| Stem | Aqueous | |||
| Leaf | Aqueous | 1% | ||
Table 6: MIC-Minimum Inhibitory Concentration of extract against E. coli and B. cereus.
DISCUSSION
Due to their phytochemical composition, which substantially contributes to their antibacterial activities, plant extracts have been found to have a variety of protective effects. In Pinda concanensis there is first report on total content of phenolic, total content of flavonoid, total content of alkaloid, total content of terpenoid and total content of saponin, with reference to these chemical compounds methanolic extract showed high content of total phenolic, high content of total alkaloid and total terpenoid than aqueous extract. But in case of saponin and flavonoid high content of saponin was observed in aqueous extract of dry root and dry stem respectively. The other Apiaceae species extracted with methanol such as Prangos ferulacea Lindl, Prangos meliocarpoides Boiss and Prangos uechtritzii Boiss and Hausskn showed highest content of phenolic compounds. Effectiveness of the extraction is powerfully affected by the extraction method, temperature, extraction time, the composition of phytochemicals and the solvent used. Methanolic extract of Pinda concanensis showed antibacterial activity against E.coli at 100 μl concentration by zone of inhibition method. The present study used distilled water and methanol to extract bioactive compounds from Pinda concanensis. Result showed that the variation in biochemical compound content in different solvents. This is because differences in the polarity of the extraction solvents could cause a wide variation in the level of bioactive compounds in the extract. A higher extraction yield was observed of Pinda concanensis in methanolic extract than distilled water. This could be because the plant material contains high levels of polar compounds that are soluble in solvents with high polarity such as water, methanol. The highest levels of Phenolic, alkaloids and terpenoids were observed in methanolic extracts, thus resulting in the highest extraction yield of methanolic extract. This would be explained by the fact that these chemicals are more soluble in methanol than the tested distilled water. These discoveries collectively suggest that the best solvent for eradicating bioactive materials from Pinda concanensis is methanol. The biological activity of the extract is substantially impacted by the effects of extraction solvents on the extraction yield and content of bioactive components. In recent years, courtesy in plant extracts with enhanced antibacterial activity has grown and various publications on the topic have been published. One of the properties of the secondary metabolites of plants is to provide protection against pathogens such as fungi, bacteria and viruses [28]. In this work, the antibacterial activity of extracts derived from methanol and distilled water of stem, root and leaf of Pinda concanensis was investigated first time against gram positive bacteria Bacillus cereus and gram negative bacteria Escherichia coli by the micro dilution method ranges from 1% to 5% extract. Among the extract tested the methanolic extract was most effective in terms of MIC (Minimum Inhibitory Concentration) values of antibacterial activity. Methanolic fresh leaf showed best inhibitory effect against both E. coli and B. cereus. The growth of food spoilage and food borne pathogens on/in food can decrease nutritional quality of the food by consuming fat, protein and carbohydrate that are present in the food, subsequently causes food discoloration, heating, mustiness, biochemical changes, weight loss and toxicity. Some species of them are able to produce highly toxic compounds which can adversely affect the health of humans. However, the safety issues with chemical preservatives are garnering more attention and natural food preservatives have great potential for the food industry. It has been reported first time that such materials as alkaloid, saponin and terpenoid can be obtained from Pinda concanensis. These active components produce varied pharmacological effect like Saponin showed anti-inflammatory antifungal, antibacterial, virucidal activity. Terpenoid exhibit antitumor, anti-inflammatory, antibacterial, antiviral and antimalarial effect. Alkaloid also showed antibacterial activity. Anticancer, antibacterial, antiviral, antioxidant and antifungal activity. From above observations the Pinda concanensis will be used in the food business not only makes antimicrobial activities easier, but it also helps with pharmacological activities like food preservation, healthcare and food nutrition. Consequently, it is a natural food additive with a large market potential [29,30].
CONCLUSION
In this research we focused on extracts obtained from Pinda concanensis in two different solvent like methanol and water were analyzed for phytochemical assay and antibacterial activity against gram positive Bacillus cereus and gram negative bacteria Escherichia coli. Results indicate that total content of phytochemicals were found more in methanolic extract than aqueous extract. MIC of gram positive and gram negative bacteria also found in methanolic extracts and aqueous extracts showed poor antibacterial activity. Therefore we can conclude that the methanol is the best solvent for extraction of phytochemicals and antibacterial activities. This antimicrobial activity may be due to presence of alkaloid terpenoid and saponin. Numerous medical plant extracts have been utilized for food preservation and medicinal purposes since they are known to have antibacterial effects. The extracts produced in this work using methanol and distilled water exhibit potent antimicrobial activity against food deterioration and food borne pathogens as well as a wide antibacterial range.
ACKNOWLEDGMENTS
The KSW gratefully acknowledge Dr. R.V. Gurav Head, Department of Botany, Shivaji University and Kolhapur for providing the necessary facilities for the study.
DECLARATION OF INTERESTS
The authors declare that they have no conflicts of interests.
REFERENCES
- Lillo A, Carvajal-Caiconte F, Vital W, et al. Braz J Biol. 2021;83:e248063.
- Ahmed J, Guvenç A, Coskun M, et al. Turk J Biol. 2011;35(3):353-360.
- Thawabteh A, Juma S, Bader M, et al. Toxins. 2019;11(11):656.
- Bagghi AK. J Ind Med Assoc. 2000;98(6):332-333.
- Cheok CY, Salman HA, Sulaiman R. Food Res Int. 2014;59:16-40.
- Bay DH, Sagdic O, Ozkan G, et al. Food Control. 2004;15(3):169-172.
- Yang CR, Zhang Y, Jacob MR, et al. Antimicrob Agents Chemother. 2006;50(5):1710-1714.
- Christova-Bagdassarian VL, Bagdassarian KS, Atanassova MS. Mintage J Pharm Med Sci. 2013;2(4):26-31.
- Truong DH, Nguyen DH, Ta NT, et al. J Food Qual. 2019;2019:1-9.
- Foye WO, Lemke TL, Williams DA. Foye’s principles of medicinal chemistry. 6th Edition, Lippincott Williams and Wilkins. Philadelphia, 2008;44-45.
- Thakur HA. Ann Plant Sci. 2017;6(12):1886.
- Hiai S, Oura H, Hamanaka H, et al. Planta Med. 1975;28(2):131-138.
- Jain A, Surana S, Gokhale SB, et al. Iran J Pharm Res. 2010;20(2):131-133.
- Kumar B, Vijaykumar M, Govindrajan R. J Ethnopharmacol. 2007;114(2):103-113.
- Kuspradini H, Wulandari I, Putri AS, et al. F1000Res. 2018;7:1839.
- Luximon-Ramma A, Bahorum T, Soobrattee MA, et al. J Agric Food Chem. 2002;50(18):5042-5047.
- Marino M, Bersani C, Comi G. Int J Food Microbiol. 2001;67(3):187-195.
- Mengoni F, Lichtner M, Battinelli L, et al. Planta Med. 2002;68(2):111-114.
- Mukherjee PK, Constance L. Kew Bull. 1986;41(1):223-229.
- Turkmen N, Sari F, Velioglu YS. Food Chem. 2006;99(4):835-841.
- Do QD, Angkawijaya AE, Tran-Nguyen PL. J Food Drug Anal. 2014;22(3):296-302.
- Rota MC, Herrera A, Martinez RM, et al. Food Control. 2008;19(7):681-687.
- Sayyah M, Hadidi N, Kamalinejad M. J Ethnopharmacol. 2004;92(2-3):325-329.
- Sindambiwe JB, Calomme M, Geerts S, et al. J Nat Prod. 1998;61(5): 585-590.
- McDonald S, Prenzler PD, Antolovich M. Food Chem. 2001;73(1):73-84.
- Oyekunle MA, Aiyelaagbe OO, Fafunso MA. Afr J Biotechnol. 2006;5(23):2405-2407.
- Sökmen M, Serkedjieva J, Daferera D, et al. J Agric Food Chem. 2004;52(11):3309-3312.
- T Ngo TV, Scarlett CJ, Bowyer MC, et al. J Food Qual. 2017;2017.
- Yang W, Chen X, Li Y, et al. Nat Prod Commun. 2020;15(3):1934578X20903555.
- Wolfe K, Wu X, Liu RH. J Agric Food Chem. 2003;51(3):609-614.