Original Articles: 2021 Vol: 13 Issue: 10
Pharmaceutical Evaluation of Some Commercial Brands of Metronidazole 500 mg Tablets Marketed in Tripoli Libya
Abstract
Metronidazole is an anti-protozoal drug which is also effective against anaerobic bacteria. The availability of several brands of metronidazole tablets in Libyan pharmacies today places health practitioners and a pharmacist in a problem of drug substitution in case of a particular brand is not available. The aim of the present study was the evaluation and comparison of pharmaceutical equivalence of five different metronidazole coated tablets 500 mg, which are commercially available in the private pharmacies in Tripoli city, produced by various pharmaceutical companies with different trade names. The pharmaceutical quality of five brands of metronidazole tablets was analyzed using official and unofficial quality control tests prescribed in different Pharmacopoeia including uniformity of weight, thickness, hardness, disintegration time, drug content as well as dissolution rate and assay. Acceptable external features as well as uniformity in diameter and thickness were revealed for all the tablets. The entire selected brands complied with the official specifications for uniformity of weight, hardness and disintegration, they released more than 75% of their drug content within 45 minutes. It can be concluded that all the brands could be regarded as bioequivalent and therefore can be interchanged in the clinical practice; this sort of study is good indicator for the evaluation of the idealness of commercial products and showed the importance of post marketing investigation for the drugs imported and distributed in Libya.
Keywords
Metronidazole; Coated tablets; Quality control evaluation; Pharmaceutical equivalence
Introduction
Tablets are oral solid unit dosage form containing a blend of active medical substances in combination with suitable excipients which are added to provide desired properties that influence their effectiveness and stability [1]. Tablet excipients must be compatible with each other and with the drug and must improve tablet stability and pharmaceutical quality. Coated tablets are tablets coated with an inert substance which resist dissolution in gastric juice, but freely dissolves and liberates the drug in the intestines [2,3]. After oral administration of drugs, it may have ineffective drug absorption due to improper dissolution rather than absorbed rapidly and completely into the blood stream [4].
Metronidazole is chemically 1H-Imidazole-1-ethanol, 2-methyl-5-nitro; 2-methyl-5-nitroimidazole-1-ethanol with chemical structure shown in Figure 1 [5]. It is a nitro-imidazole derivative that has been synthesized in various laboratories throughout the world [6]. It is effective against protozoal infestations and bacterial infections, it has been used for the treatment of infections for more than 45 years and also used during pregnancy, bacterial vaginosis and prophylaxis against anaerobic infection after bowel surgery, wound abscess, antibiotic associated colitis against Helicobacter pylori and Giardia lamblia that can cause travelling diarrhea [7,8]. It’s a white or yellowish microcrystalline powder, slightly soluble in water, acetone, alcohol and methyline chloride [9]. The disposition of metronidazole in the body is similar for both oral and intravenous dosage forms. Following oral administration, metronidazole is well absorbed, with peak plasma concentrations occurring between 1-2 hours after administration. Plasma concentrations of metronidazole are proportional to the administered dose. Oral administration of 500 mg tablet produced peak plasma concentrations of 12 mcg/ml. Studies reveal no significant bioavailability differences between males and females; however, because of weight differences, the resulting plasma levels in males are generally lower [10].
In Libya, there are many different brands of metronidazole tablets available from different multinational companies. Each brand has its own formulation which affects the release and delivery of drug and produce variable clinical responses. Evaluation of in vitro release and the physicochemical properties of these brands are very important as it can be used to evaluate the bioavailability and pharmaceutical equivalence [11]. Various brands available in the market are considered pharmaceutically equivalent if they contain the same amount of active ingredients in the identical dosage form and meet the same compendia standards in strength, quality, purity and identity but may differ in shape, packaging, excipients, expiration time and labeling requirements [12]. According to World Health Organization (WHO) the prevalence of fake medicines was higher in developing countries with week regulations, enforcement, and scarcity of supply of essential medicines, unregulated market and unaffordable prices [13]. For these reasons, the safety, quality and efficacy of drug products especially in developing counties cannot be granted, therefore post market qualitative studies are important, few drug quality control studies have been conducted so far in Tripoli Libya, these studies encouraged to examine the medicine quality for continuous monitoring and control of drug products in the market that might prevent the prevalence of counterfeits and substandard medicines and ensure the use of medicines of standard quality [14-17].
Quality control is a procedure or set of procedures intended to ensure that a manufactured product is complied with specifications. Quality control has an important role in pharmaceutical field, it’s an investigation applied to drug and drug product, including all those factors which contribute directly or indirectly to the safety, effectiveness and reliability of product. Patient safety is the most guiding value in all drug factories; the basic goal is to provide efficient, safe and compatible products for the prevention and treatment of illness. It’s important to promote health and quality of life with product by sharing guidance between consumers and healthcare professionals on the correct proper use and storage of the products [18].
This study was conducted to evaluate and assess the pharmaceutical quality of different metronidazole 500 mg tablets available in private pharmacy of Tripoli Libya. The assessment included the evaluation of weight uniformity, diameter, thickness, hardness, dissolution, disintegration, identification, assay and price of tablets, to ascertain that all the brands under investigation are pharmaceutically equivalent.
Materials and Methods
Metronidazole tablets having label strength of 500 mg of five different brands were purchased from different private pharmacies in Tripoli Libya. The products were coded as A, B, C, D and E as illustrated in Table 1 and the study was performed within product expiration dates.
Distilled water, 0.1 N hydrochloric acid, chloroform, acetone, acetic anhydride, anhydrous acetic acid, perchloric acid, brilliant green and pure sample of metronidazole (RTC) lot No. p500052 was obtained from the National Centre for Food and Drug Control.
Evaluation tests
Visual inspection: Samples of 20 tablets from each batch were selected randomly and inspected for their external characteristics such as color, surface texture and shape, presence of grooves; monograms and coat were described based on the visual observation.
Weight uniformity: Twenty tablets of each product code were weighed using an electronic digital balance, each tablet was weighed individually then the average weight was calculated for each brand. Tablets were examined for their uniformity of weight and the percentage deviation allowed by USP generally ± 10% for tablets weighing 130 mg or less, ± 7.55 for tablets weighing more than 130 mg to 324 mg and ± 5% for tablets weighing more than 324 mg [19].
Hardness and tablet dimensions: Hardness, thickness, and diameter of samples of 20 tablets were determined using tablet combination tester (Erweka TBH 320 WTD Multi-Check tester, Germany). In the hardness test, pressure was applied on the tablet and the force caused the tablet to break up was recorded. The optimum hardness regarded for coated tablets is 10-20 kg/cm2. Tablet thickness and diameter should be controlled within a ± 5% of a standard value. [20,21]
Disintegration Test: Samples of six tablets were selected from each brand. Tablets were placed in six tubes of the basket-rack assembly of the disintegration time tester PTZ Auto 1EZ (Pharma test, Germany) and perforated cylindrical plastic discs were put on top surface of each tablet. The assembly was allowed to move up and down in a beaker containing 1 litre of distilled water at 37 ± 0.5°C. The time taken to break each tablet into small particles and pass out through the mesh at the bottom of the tube was recorded. Mean disintegration time was calculated for each one of the brands.
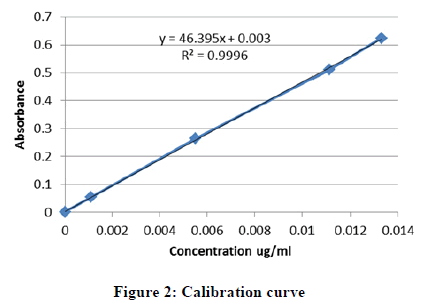
Preparation of the standard curve: A stock solution of pure metronidazole was prepared by dissolving 50 mg of pure metronidazole in 50 ml of 0.1 N HCl to produce a 1 mg/ml solution. 10 ml of stock solution was transferred to a measuring flask and the volume was made to 100 ml with 0.1 N HCl. Five different concentrations of pure metronidazole were prepared from stock solution. The absorbance of 6 concentrations including blank was taken at 278 nm using UV-spectrophotometer and calibration curve was plotted (Figure 2).
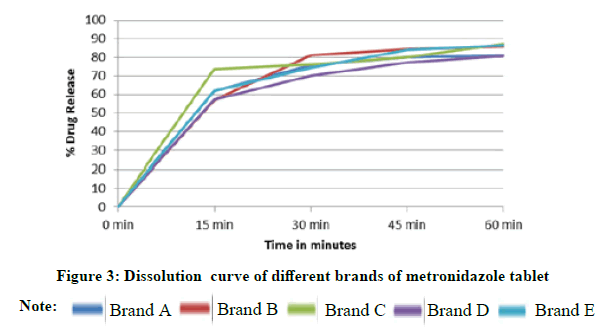
Dissolution rate determination: Dissolution test was carried out by a dissolution apparatus which operated at 100 rpm for 60 min, using 0.1 N Hydrochloric acid (900 ml) prepared as a dissolution medium, at 37°C ± 2°C. Six tablets from each brand were placed in a small wire mesh basket fastened to the bottom of the shaft connected to a variable speed motor. Samples withdrawn at intervals of 15, 30, 45, and 60 minutes, on each time, samples were diluted at first by withdrawn 5 ml replaced with fresh medium in 25 ml capacity flask, then withdrawn from it 1 ml and dilute it again by fresh medium in 25 ml capacity flask, samples were filtered and absorbance measured at 278 nm using pure medium as a blank. Percentage of average drug release for each brand was plotted against time (Figure 3).
Assay of metronidazole tablets: Twenty tablets were weighed and powdered. 0.2 g of metronidazole powder were transferred to sintered-glass crucible and extracted with six 10 ml quantities of hot acetone. After cooling it was added to combine extract of (50 ml of acetic anhydride and 0.1 ml of a 1% w/v solution of brilliant green in anhydrous acetic acid) and titrated with 0.1 M perchloric acid vs. a yellowish green end point. This operation was repeated without the powdered tablets. The difference between the titrations represents the amount of perchloric acid required. Each ml of 0.1 M perchloric acid vs. is equivalent to 17.12 mg of C6H9N3O3 [21].
Identification: A quantity of powdered tablets containing 0.1 g of metronidazole was shaken with 40 ml of chloroform for 15 min, after filtration the filtrate was evaporated to dryness, the infrared absorption spectrum of the residue, was concordant with the reference spectrum of metronidazole [21].
Results and Discussion
Five commercial metronidazole 500 mg tablets (Table 1) were assessed for their pharmaceutical quality according to the described requirements that are stated in the official compendia. The evaluation tests were performed on the samples while in their intended shelf life. The apparent physical characteristics of the samples based on visual inspection were described in Table 2. All tablets were elegant attractive appearance with smooth surface texture, biconvex and round in shape except brand that was oval, with uniform white colours except brand E that was bright yellow in colour. Brand D did not have monograms or score lines as other brands which were marked on the surface with symbols indicating the drug name or strength and the company name or logo for further product identification, there were no defects in the tablets coat integrity.
| Product code | Batch No. | Manufacture Date | Expire Date | Price/tablet in LD |
|---|---|---|---|---|
| A | 0106 | 3/2015 | 3/2020 | 1.2 |
| B | 8KV656 | 6/2018 | 6/2021 | 0.7 |
| C | 8EG017 | 2/2018 | 1/2021 | 0.7 |
| D | 73938 | 10/2017 | 10/2022 | 0.5 |
| E | 5535 | 2/2018 | 2/2022 | 0.3 |
Table 1: Label information of five different brands of metronidazole tablet 500 mg under investigation
| Parameter | Brand A | Brand B | Brand C | Brand D | Brand E |
|---|---|---|---|---|---|
| Shape and color | Oval white | Rounded white | Rounded white | Rounded white | Rounded bright yellow |
| Surface texture and Convexity | Smooth and biconvex | Smooth and biconvex | Smooth and biconvex | Smooth and biconvex | Smooth and biconvex |
| Monograms and score lines | Yes | Yes | Yes | No | Yes |
| Defect in the tablet coat | No | No | No | No | No |
Table 2: Appearance features of the different brands of metronidazole 500 mg tablets
All brands of metronidazole tablets were consistent in their weight and exhibited uniform geometrical dimension parameters (Table 3) the deviation of the tablets weight from the average weight were in the permitted limit with a deviation less than ± 5%. Brand B and D exhibited quite similar average weight and all the investigated brands demonstrated similar diameters and thickness except brand A that showed to be the largest in average weight, diameter and thickness as well as the most expensive one among the selected brands. The hardness test results (Table 3), showed that brand A exhibited greater capability to resist chipping, while brand B and D demonstrated the lowest and weakest solidity in comparison to the other brands.
| Brand | Average weight g | Weight variation % | Dissolution % | Hardness (N) | Disintegration time (min) | Assay (%) | Diameter (mm) | Thickness (mm) |
|---|---|---|---|---|---|---|---|---|
| A | 0.9605 | ± 1.96 | 81.06061 | 202.7 | 00:06:34 | 105% | 18.031 | 6.903 |
| B | 0.6664 | ± 2.41 | 85.60606 | 130.935 | 00:05:17 | 103.50% | 12.627 | 5.602 |
| C | 0.7047 | ± 1.57 | 87.12121 | 157.065 | 00:06:27 | 99.20% | 13.14 | 5.3455 |
| D | 0.6714 | ± 2.15 | 80.68182 | 130.055 | 00:07:07 | 96.70% | 12.73 | 5.3605 |
| E | 0.7548 | ± 3.85 | 86.36364 | 183.085 | 00:03:56 | 94.10% | 13.174 | 5.516 |
Table 3: Evaluated physicochemical parameters of the five brands of metronidazole tablets
All brands passed the disintegration time test according to the official limit. Tablets were broken up and disaggregated in to their original granules and particles within 15 minutes. Brand E demonstrated very rapid disintegration time compared to the other brands (Table 3). While brand D showed a more prolonged disintegration time (7 minutes). It was found that all brands were in compliance with the standard limit for dissolution test (Figure 3). The drug release values were more than 80% in one hour, all the assessed brands exhibited similar patterns of drug dissolution excluding brand C which had the fastest drug release with more than 70% in 15 minutes. The results obtained from the evaluation of active ingredients content were within the limits (95%-105%) results were showed in Table 3.
Figures 4 to 9 shows the IR spectrum of different commercial brands of metronidazole and the standard. It was observed that all spectra obtained for different samples of metronidazole have the similar absorption bands with the IR spectrum of standard metronidazole. The similarity between the spectra is a strong indicative of the identity of metronidazole in all of the samples analyzed using IR technique, as shown below the characteristics peaks of brand C was the most different from the standard.
Conclusion
As shown in this study, all brands of metronidazole 500 mg tablets available in local market of Tripoli Libya complied with USP and BP standards, and can be interchangeable while there was no significant variation in the quality of the tested drugs, it can be inferred that the brands tested of metronidazole tablets are pharmaceutically equivalent. This study highlights the need for focusing on the post marketing evaluation of pharmaceutical products circulating in the markets originated from different manufacturers especially in developing countries.
Acknowledgment
The authors would like to thank the National Centre for Food and Drug Control, Tripoli for the generous help, technical support of sample analysis and facilities provided.
References
- Aulton ME, Aulton S. 2007.
- Ansel HC. Philadelphia. 1981.
- Perumalla SR, Sun CC. J Pharm Sci. 2014; 103(4): 1126-1132.
- Banker GS, Rhodes CT. J Pharm Chem. 1990: 23.
- Wilson G. J Chem Med. 1998: 211-212.
- Safdar KA, Naqvi SBS, Gauhar S, et al. J Pharm Sci. 2014; 3(12): 1458-1470.
- Adil M. J Chem Pharm. 2016.
- USP Pharmacopeia. 2014.
- Itiola OA, Pilpel N. Pharmazei. 1996; 51: 987-989.
- Mosby’s Medical Dictionary. 9th edition. 2009.
- Food and Drug Administration. 2004.
- World Health Organization. 2017.
- Elghnimi TY, El-majri MA, Bennouh R, et al. Int J Pharm Sci Res. 2016; 7(6): 2402-2409.
- Elghnimi TY, Bzezi W, Siaan MM, et al. Eur J Biomed Pharm Sci. 2019; 6 (6): 138-143.
- Siaan MM, Anwair MAS, Sadek F, et al. J Chem Bio Phy Sci. 2017; 7(4): 857-867.
- Zaek AS, Benhamed BA, Alshahomy MA, et al. Chem Bio Res. 2019; 2: 06-12.
- Management of Product Responsibility. Orion.
- Lieberman HA, Lachman L. J Chem Pharm. 1981; 2: 249.
- Gennaro AR, Remington. J Pharm. 1995.
- British Pharmacopoeia Appendix: XII. 2002: A2-53.
- The International pharmacopoeia. 9th Edition. 2019.