Original Articles: 2024 Vol: 16 Issue: 5
Nutritional Composition, Phytochemical, Total Phenolic Content and Lipid Peroxidation Inhibition of Banana Blossom of Musa sapientum Linn. (Musa ABB group, cv. Namwa)
Suwanna Promthong*
Department of Agricultural Technology, Ramkhamhaeng University, Bangkok, Thailand
- Corresponding Author:
- Suwanna Promthong
Department of Agricultural Technology, Ramkhamhaeng University, Bangkok, Thailand
Received: 29-Apr-2024, Manuscript No. JOCPR-24-122015; Editor assigned: 02-May-2024, PreQC No. JOCPR-24- 122015 (PQ); Reviewed: 16-May-2024, QC No. JOCPR-24-122015; Revised: 23-May-2024, Manuscript No. JOCPR- 24-122015 (R); Published: 05-Jan-2024, DOI:10.37532/0975-7384.2023.16(5).154
Citation: Promthong S. 2024. Nutritional Composition, Phytochemical, Total Phenolic Content and Lipid Peroxidation Inhibition of Banana Blossom of Musa sapientum Linn. (Musa ABB group, cv. Namwa). J. Chem. Pharm. Res. 16:154.
Copyright: © 2024 Promthong S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, providedthe original author and source are credited
Abstract
This study aimed to investigate the nutritional composition, phytochemical, total phenolic content, and lipid peroxidation inhibition of banana blossom (Musa sapientum Linn.) in Thailand. The collected banana blossoms were separated into three parts; bract, floret, and young blossom, which each part was dried and extracted by maceration of 95% ethanol and decoction with water. The results showed that all parts of it contained high nutrients; crude fiber, crude protein, crude fat, total ash, total carbohydrate, calcium, and phosphorus, which bract possessed the greatest fiber (28.79% DM), followed by young blossom (21.14% DM) and floret (19.58% DM) (P<0.05). Furthermore, banana blossom indicated the presence of alkaloids, tannins, saponins, terpenoids, flavonoids, phenolic compounds, and anthocyanins. The floret ethanol extract showed the greatest total phenolic content (168.88 mg GAE/g), followed by bract water extract (147.27 mg GAE/g), bract ethanol extract (145.12 mg GAE/g), floret water extract (120.11 mg GAE/g) and young blossom water extract (114.45 mg GAE/g), while the least was young blossom ethanol extract (86.07 mg GAE/g) (P<0.05). Lipid peroxidation inhibition of banana blossom water extract showed higher potential than 95% ethanol extract of its all parts (P<0.05). Thus, this study found that all parts of it contained high nutrient compositions, potential source of natural antioxidants, high level of total phenolic content, and high lipid peroxidation inhibition. Total phenolic contents were correlated with inhibition of lipid peroxidation activities. The banana blossom and its parts possess the potential as a source of natural antioxidants that benefit natural medicine and therapeutic food including oxidative stress reduction. Therefore, banana blossom and its parts possess the potential benefits as dietary supplementation and antioxidant for human and animal health.
Keywords
Banana blossom; Musa sapientum Linn; Nutritional composition; Phytochemical; Total phenolic content; Lipid peroxidation inhibition
Introduction
Banana is a type of monocotyledon plant belonging to genus Musa and family Musaceae, which can be divided into different groups based on chromosome numbers and ploidy as well as the characteristics of the two wild Banana are types of monocotyledon plants belonging to genus Musa and family types: Musa acuminata (genome type A, AA) and Musa balbisiana (genome type B, BB) [1].
These two varieties are primitive edible banana and they are endemic to Southeast Asia and Western Pacific region. These are important species used for developing banana cultivars that give rise of the various major genomic groups [2]. There are two specific names for edible bananas, Musa paradisiaca and Musa sapientum. Musa sapientum referring to ‘banana’ which has small fruits known as their edible fruits and Musa pardisiaca referring to ‘plantains’ which has bigger size and higher starch fruits for cooking [3].
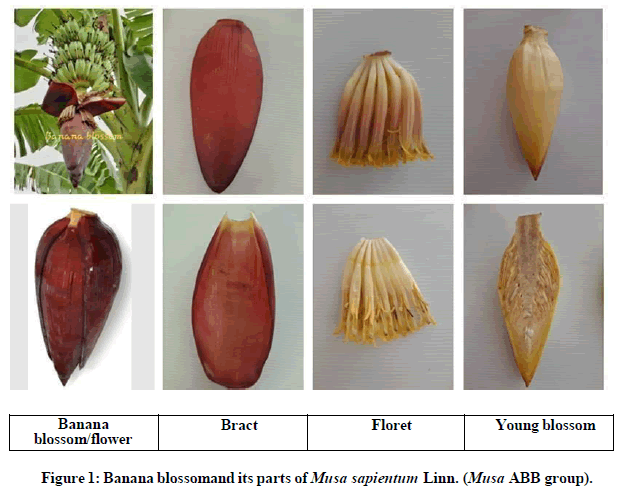
The banana of Musa sapientum Linn. (ABB genome group, Kluai Namwa; Kluai is the Thai word for banana) cultivar is a hybrid variety by natural crossing of two wild cultivars of Musa acuminata and Musa balbisiana [4,5]. Kluai Namwa (Musa ABB group) is well known and widely consumed throughout Thailand for its edible fruits and cooking. Each banana plant normally develops only one inflorescence which commonly called “blossom”. The banana plant produces inflorescence at the end of the plant and it grows from the top of the parent plant which extends from the pseudostem. Banana inflorescence, a dark purple-red heart shaped structure, is a by-product of banana cultivation. It is commonly called “banana blossom”, “banana flower”, “banana male bud”, or “banana heart” which is actually the sterile, male flower of the plant and it’s called “Huaplee” in Thai language. The banana blossom is enclosed by many curved bracts, which are dark violet and red color. The bracts unfold from the base to the top and fall off after the floret developing intofruits. The banana inflorescence consists of several florets and there are ovaries at the base and anthers at the top of florets.Banana blossoms were the byproducts of banana cultivation; they were consumed as a vegetable either raw or cooked, and used for Thai traditional medicine and Thai health food as a preparation for postpartum breastfeeding care [6], and antidiabetic activities.
Banana blossom has nutritional value and it is consumed as an additive in several Asian countries such as Thailand, Sri Lanka, and Indonesia, and etc. In Thailand, banana blossom is usually considered as vegetable and a functional food because it is a good source of nutritional value as it commonly contains carbohydrates, proteins, lipids, fibers, some vitamins and minerals for example, calcium, potassium, magnesium, iron, copper, vitamin A,C,E, and natural phytonutrients [7-9], such as saponins, flavonoids, phenols, glycosides, tannin and steroids [10]. It has rich dietary fiber, which could be found in both outer and inner bracts [11]. Moreover, it contains high quality protein because of its well balance essential amino acid referring to high dietary fiber and flavonoid concentrations [12].
Banana blossom has nutritional value and health effects that it is generally used for health problems, e.g., heart attack, diarrhea, asthma, and stomach pain. It could also be used to treat bronchitis, dysentery, weight loss, anemia, constipation, ulcers, menstruation, diabetes, cancer, and etc. [13]. Also, it possesses several biological activities, such as antioxidant a anti-inflammatory, antimicrobial activities, antidiabetic activities, antiulcer, anticancer activities and anti-HIV activities [9,10]. Interestingly, many plant parts such as fruits, vegetables, flowers, and leaves have been shown to protect against various diseases [14], which may be due to the presence of high amounts of phenolic antioxidants [15]. Because banana blossom possesses various medicinal benefits, so that this plant is actually made as the significant functional food [16]. Banana blossom could be a major source of antioxidants that can scavenge free radicals and confirm protection oxidative stress, including antidiabetic activity [17-19]. Although banana blossom has nutritional properties and health benefits, however, there are few studies have analyzed each part of banana blossom for nutritional compositions, phytochemical constituents, and antioxidant activities, which is the scientific investigation into the mechanisms of health benefits of banana blossoms. Therefore, the objective of this study was to investigate the nutritional composition, phytochemical screening, total phenolic content, and lipid peroxidation inhibition of each part of banana blossom of Musa sapientum Linn. (Musa ABB group, cv. Namwa), which may prove to be against oxidative stress as a factor of various metabolic disorders in human and/or animal body.
Materials and Methods
Preparation of banana blossom powder
The studying banana blossoms (called “Huaplee in Thai) of Musa sapientum Linn. (Musa ABB group, cv. Namwa) were collected from local plantations of six areas in Thailand, and the banana blossoms of each area were weighed separately. The fresh banana blossom was separated into three parts consisting of bract (outside bract with dark violet and red colors), floret (inside each bract with anthers and ovary), and young blossom (inner banana blossom that still young and soft with white color) showing in Figure 1. Each part of banana blossom was chopped into small pieces, and washed inmixed water together with 0.2% citric acid for 30 minutes in order to eliminate and prevent enzymatic browning reaction. Then,after that, water was drained and the parts of the slicing banana blossom were spread in the tray and dried by using the hot air oven at 50°C, and also it was grinded after drying into fine powder. Banana blossom powder was packed in the sealedcontainers and stored at 4°C condition before uses.
Preparation of banana blossom crude extracts:
Each part of the banana blossom powder samples was extracted by using two different solvents, e.g., ethanol and water, and the two methods, like maceration and decoction were employed.
Maceration method: The bract powder, floret, young blossom, and mixed parts (3 parts of blossom said earlier) powder were macerated in 95% ethanol at a ratio sample:solvent of 1:10 for 7 days at room temperature, then filtered with filter paper (Whatman No.1) and its residue was macerated twice.
Decoction: The bract powder, floret, young blossom, and mixed parts powder were broiled in water at 90°C for 1 hour, then each part was filtered, and its residue was boiled twice.
The combined extracts were concentrated using a rotary evaporator at 50°C and dried in the hot air oven at 50°C in order to obtain crude extracts. After that, the percentages of components were calculated and lastly, the obtained crude extracts were stored at -20°C before uses.
Nutritional composition of the banana blossom powder samples:
Samples of bract powder, floret, young blossom, and mixed parts powder were analyzed into various compositions, such as moisture, crude protein, crude fat, crude fiber, total ash, acid soluble ash, insoluble ash (AIA), calcium, and phosphorus by using the standard methods published by Association of Official Analytical Chemists [20,21]. To calculate moisture (Dry Matter; DM), samples were dried in the hot air oven at 95°C-100°C until its weight being constant (method 934.01). Crude protein (N x 6.25) was determined by the Kjeldahl method (method 976.05). Crude fat (as Ether Extract; EE) was determined using the Soxhlet extraction method with petroleum ether as solvent (method 935.38). Crude ash was measured by incineration in a muffle furnace at 550°C-600°C (method 942.05). Crude fiber was determinedas the residue after sequential treatment with 1.25% H2SO4 and with 1.25% NaOH (962.09). Calcium was performed using titration method against potassium permanganate (method 927.02) and phosphorus was determined using photometric method (method 965.17) with molybdovanadate reagent and determination using UV-VIS spectrophotometer.
All the estimations were performed in triplicates. Carbohydrates were calculated as Nitrogen-Free Extract (NFE) and total carbo-hydrate content was determined using the method according to Steenfeldt and Pettersson [20]. NFE supposedly represents the soluble carbohydrates of food/feed, i.e., starch and sugar. Total carbohydrates include all three types of carbohydrates (crude fiber, sugar starch) found in food/feed. Each of these types of carbohydrate is seriously critical to sufficient energy intake and overall health [21].
%NFE (carbohydrate) = 100 - (%Moisture + %Crude protein + %Crude fat + %Crude ash + %Crude fiber) (Eq…1)
%Total carbohydrate content = 100 - (%Moisture + %Crude protein + %Crude fat + %Crude ash) (Eq…2)
Method for qualitative phytochemical screening
Preliminary qualitative phytochemical screening of the ethanol extracts and water extracts of each part of the banana blossom was subjected by using the methods described by Savithramma et al., [22], with a slightly modifying. Each of dried extracts was weighed (1 g), then it was dissolved in 100 mL of solvents, ethanol, and water, and these were also filtered. These processes were the preparation of stock solution for phytochemical screening [23].
Test for phenolic compounds (Lead acetate test)
Three ml of extract solution were taken in a test tube, while 1 ml of 10% lead acetate solution was added. After a few minutes, appearance white precipitate appeared which indicates the presence of a phenolic compound.
Test for flavonoids (Alkaline reagent test):
One mL of extract solution was taken in a test tube and added few drops of 2% sodium hydroxide solution. An intense yellow color appeared in the test tube and became colorless after adding of a few drops of dilute hydrochloric acid, which indicates the presence of flavonoids.
Test for anthocyanins (HCL test):
Two mL of extract solution was taken in a test tube and added to 2 mL of 2 N hydrochloric acid solutions. The appearance of a pink-red color which turns blue-violet after the addition of ammonia indicates the presence of anthocyanins.
Test for tannins compounds (Ferric chloride test):
Two mL of extract solution was taken in a test tube and added few drops of 1% ferric chloride (FeCl3) solution (light yellow). Then, it was for brownisobservedh-green or blue-black coloration, which indicates the presence of tannin compounds.
Test for alkaloids (Wagner’s test):
One mL of extract solution was added up with a few drops of Wagner’s reagent (iodine in potassium iodide), carefully mixed, and checked the formation of brown/reddish precipitate indicates the presence of alkaloids.
Test for saponins (Frothing test and Olive oil test):
Two mL of extract solution were mixed with 20 mL of distilled water and rapidly shaken for 15 minutes. If the formation of foam layer height greater than 1 centimeter, which indicates the presence of saponins. Observed for a stable persistent froth, the frothing was mixed with 3 drops of olive oil and rapidly shaken. The formation of an emulsion yields a positive result [24].
Test for cardiac glycosides (Keller-Killiani Test):
One mL of extract solution was taken in a test tube and added up with 1.5 mL of glacial acetic acid solution, including added one drop of 5% of ferric chloride solution, then 1 ml of concentrated sulfuric acid was carefully added along the side of test tube. A blue colored solution which shows the presence of cardiac glycosides.
Test for steroids (Salkowski test):
One mL of extract solution was added 10 mL of chloroform and 1 mL of concentrated sulfuric acid around the inside the surface of the test tubes. Whenever the upper turns red and sulfuric acid layer shows greenish yellow fluorescence.This indicates the presence of steroids.
Test for terpenoids (Salkowski test):
In a test tube containing 2 mL of chloroform, 0.5 mL of extract solution was added. Then, concentrated sulfuric acid (3 mL) was carefully added to form a layer. If a red-dish-brown coloration of the interface was occurred, it indicates the presence of terpenoids.
Test for anthraquinones (Borntrager’s test):
A few drops of concentrated sulfuric acid were added to 1 mL of extract solution. Then, added 10 mL of 10% ammonia solution. The appearance of a rose-pink color indicates the presence of anthraquinones.
Antioxidant activity of banana blossom extract:
Total phenolic contents by Folin-Ciocalteu reagent method:
The Total Phenolic Content (TPC) of extracts was determined for individual extracts using the Folin-Ciocalteu colorimetric method modified by Seladji et al., [24]. The Folin-Ciocalteu reaction is an antioxidant assay based on electron transfer that measures the reductive ability of an antioxidant. It is a method used to determine the total phenol orpolyphenol content of plant derived food and biological samples [25]. From each dried crude extract 1 mg was dissolvedin ethanol or water 1 mL (1 mg/mL). In addition, 1 mL of each extract solution was mixed with 5 mL of 10% (w/v) Folin-Ciocalteu reagent. After that, the test tube was kept in a dark place for 5 minutes. Lastly, 4 mL of 7.5% sodiumcarbonate (Na2CO3) was added to the solution and mixed well. After 30 minutes of incubation in a dark condition at room temperature. The absorbance was measured against a blank without extract at 765 nm (UV-Vis Spectrophotometer). Gallic acid was used for establishing the standard curve and the total phenolic content was expressed as milligrams of Gallic Acid Equivalents per gram (mg GAE/g) of crude extract.
Inhibition lipid peroxidation:
A modified Thiobarbituric Acid Reactive Substances (TBARS) assay was used to measure the lipid peroxide formed, using egg yolk homogenates as lipid-rich media, as described by Upadhyay et al., [26]. Briefly explain, 0.5 mL of egg yolk homogenate (10% in cold phosphate buffer saline pH 7.4, v/v) was added to 0.1 mL of the extract in a test tube. The volume was then made up to 1.0 mL with distilled water. Thereafter, 0.05 mL of ferrous sulfate (FeSO4) (0.07 M) was added and the mixture was incubated at 37°C for 30 min, to induce lipid peroxidation. Then, 1.5 mL of 20% acetic acid (pH 3.5), followed by 1.5 mL of 0.8% Thiobarbituric Acid (TBA) (w/v) (prepared in 1.1% sodium dodecyl sulfate,SDS) and 50 μL 20% Trichloroacetic Acid (TCA) were added, vortex mixed and heated at 95°C for 1 hour. After cooling, 6mL of 1-butanol was added and the mixture was centrifuged at 3,000 rpm for 10 minutes. The absorbance of the organic upper layer was measured at 532 nm by a spectrophotometer. For the blank 0.1 mL of distilled water was used in place of the extract. The degree of lipid peroxidation was assayed by estimating the TBA Reactive Substance (TBARS) content. The percentage inhibition was calculated with this following formula:
% Inhibition of lipid peroxidation = (Acontrol-Asample)/Acontrol x 100 (Eq…3)
Where Acontrol is the absorbance of the control and Asample is the absorbance of the test samples/standard.
Statistical analysis
Analysis of Variance (ANOVA) was used for statistical analysis and Duncan’s Multiple Range Tests (DMRT) was used to compare the significant differences between group means. Statistical significance was determined at P<0.05. The result data will be expressed by the mean ± Standard Deviation (SD) [27].
Results
The components of banana blossom
The Each part of the fresh banana blossom of Musa sapientum Linn. (Musa ABB group, cv. Namwa) was dried and the components of each part of banana blossom were shown in Table 1. Fresh banana blossom contained 93.05% ± 0.31% moisture, which the percentage components of each part of the dried banana blossom were bract 2.05% ± 0.34%, floret2.15% ± 0.09%, young blossom 2.75% ± 0.42%, and mixed parts 6.95% ± 0.31% of fresh weight. In addition, the calculated percentages of dry weight of bract, floret, and young blossom were 29.43% ± 0.46%, 30.99% ± 0.21%, and 39.57% ± 0.56%,respectively.
| Moisture of Fresh blossom (%) | Dried blossom (% Fresh weight) | Dried blossom (% Dry weight) | |||||
|---|---|---|---|---|---|---|---|
| Bract | Floret | Young blossom | Total | Bract | Floret | Young blossom | |
| 93.05 ± 0.31 | 2.05 ± 0.34 | 2.15 ± 0.09 | 2.75 ± 0.42 | 6.95 ± 0.31 | 29.43 ± 0.46 | 30.99 ± 0.21 | 39.57 ± 0.56 |
Note: Values are mean ± standard deviation of the six independent determinations.
Table 1: Percentage of components of dried blossom of banana Musa sapientum Linn. (Musa ABB group, cv. Namwa).
The yield of banana blossom extracts
The yield of each part of the banana blossom extracts of Musa sapientum Linn. (Musa ABB group, cv. Namwa), this study resulted that the percentage of dry matter were shown in Table 2. Water extracts demonstrated yields more than ethanol extracts, such as water extract and ethanol extract of mixed parts demonstrated yield 17.05 ± 3.02, and 8.97 ± 1.77%, respectively (P<0.05). The water extract of dried young blossom demonstrated the highest yield (19.94 ± 1.04%), followed by dried bract (16.07 ± 1.07%), meanwhile dried floret demonstrated the lowest yield (15.43 ± 3.26%). The 95% ethanol extract of dried bract demonstrated the highest yield (11.58 ± 1.44%), followed by dried floret (10.08 ± 1.29%) and young blossom demonstrated the least yield (9.54 ± 2.36%).
| Extracts | Bract | Floret | Young Blossom | Mixed parts |
|---|---|---|---|---|
| %Yield (Dry matter) | ||||
| Water 95% Ethanol | 16.07 ± 1.07B | 15.43 ± 3.26B | 19.94 ± 1.04A | 17.05 ± 3.02AB |
| 11.58 ± 1.44C | 10.08 ± 1.29C | 9.54 ± 2.36C | 8.97 ± 1.77C | |
Note: Values are mean ± standard deviation of the six independent determinations.
A-CThe letters indicate significant differences in extracted the parts of banana blossom (P<0.05).
Table 2: Percentage yield of water extracts and ethanol extracts of banana blossom of Musa sapientum Linn. (Musa ABB group, cv. Namwa).
The nutritional composition analysis of banana blossom samples
Each part of the banana blossom powder of Musa sapientum Linn. (Musa ABB group, cv. Namwa) was shown as the dry matter percentage of nutritional composition in Table 3. The nutrient composition of all parts of the banana blossom was found a significant difference (P<0.05). The bract showed that crude fiber, NFE, and total carbohydrate greater than the floret and young blossom, but less protein, and soluble ash. The floret showed greater crude fat and phosphorus than the bract and young blossom. The young blossom showed greater crude protein, total ash, acid insoluble ash, soluble ash, and phosphorus, but less NFE and total carbohydrate than the bract and floret (P<0.05). It could be concluded that mixed parts yielded organic matter (84.09 ± 0.96%), crude protein (13.86 ± 0.57%), crude fat (4.56 ± 0.46%), crude fiber (23.78 ± 1.50%), total ash (14.35 ± 0.77%), acid insoluble ash (0.502 ± 0.237%), soluble ash (13.85 ± 0.89%), NFE (33.68 ± 2.61%), total carbohydrate (57.47 ± 1.53%), calcium (0.068 ± 0.022%), and phosphorus (0.598 ± 0.063%). Especially, bract yielded the greatest fiber (28.79 ± 2.36%), followed by young blossom (21.14 ± 1.10%) and floret (19.58 ± 2.40%).
| Parameter | Nutritional composition (% Dry matter) | |||
|---|---|---|---|---|
| Bract | Floret | Young blossom | Mixed parts | |
| Dry matter | 91.15 ± 0.94 | 90.29 ± 1.21 | 90.35 ± 1.29 | 90.24 ± 0.69 |
| Organic matter | 85.81 ± 1.18a | 84.47 ± 1.29a | 82.22 ± 1.08b | 84.09 ± 0.96 |
| Crude protein | 7.19 ± 1.29C | 14.20 ± 0.74B | 20.09 ± 0.52A | 13.86 ± 0.57 |
| Crude fat | 3.08 ± 0.44Y | 7.15 ± 0.87X | 3.16 ± 0.28Y | 4.56 ± 0.46 |
| Crude fiber | 28.79 ± 2.36c | 19.58 ± 2.40d | 21.14 ± 1.10d | 23.78 ± 1.50 |
| Total ash | 13.02 ± 1.04E | 14.04 ± 1.07E | 16.05 ± 0.84D | 14.35 ± 0.77 |
| Acid insoluble ash | 0.232 ± 0.130y | 0.158 ± 0.065y | 0.362 ± 0.216xy | 0.502 ± 0.237 |
| Soluble ash | 12.32 ± 1.35c | 13.86 ± 1.08b | 15.54 ± 1.04a | 13.85 ± 0.89 |
| Nitrogen free extract | 39.08 ± 2.78A | 35.33 ± 1.99B | 29.88 ± 2.18C | 33.68 ± 2.61 |
| Total Carbohydrate | 67.87 ± 0.79X | 54.91 ± 2.09Y | 51.04 ± 2.24Z | 57.47 ± 1.53 |
| Calcium | 0.045 ± 0.009 a | 0.042 ± 0.016 a | 0.053 ± 0.009 a | 0.068 ± 0.022 |
| Phosphorus | 0.431 ± 0.083 Y | 0.779 ± 0.084 X | 0.784 ± 0.037 X | 0.598 ± 0.063 |
Note: Values are mean ± standard deviation of the six independent determinations, means in the same row with different superscripts are significantly different (P<0.05).
%Nitrogen Free Extract (NFE) = Carbohydrate = 100 - (%Moisture + %Crude protein + %Crude fat + %Crude ash + %Crude fiber)
%Total carbohydrate content (%) = 100 - (%Moisture + %Crude protein + %Crude fat + %Crude ash).
Table 3: Nutritional composition of banana blossom powder of Musa sapientum Lin (Musa ABB group, cv. Numwa)
Qualitative phytochemical screening
The phytochemical screening of each part extracts of banana blossom of Musa sapientum Linn. (Musa ABB group, cv. Namwa) was found and showed in Table 4. The 95% ethanol extract and water extract of the bract, floret, young blossom, and mixed parts contained the same compound of alkaloids, saponins, tannins, phenolic compounds, flavonoids, anthocyanins, and terpenoids, meanwhile steroids, cardiac glycosides, and anthraquinone glycosides were not found in all crude extracts [28].
| Phytochemicals | Testing/Reagent | Extracts | Bract | Floret | Young blossom | Mixed parts |
|---|---|---|---|---|---|---|
| Phenolic compounds | Lead acetate test | 95% ethanol | + | + | + | + |
| Water | + | + | + | + | ||
| Flavonoids | Alkaline reagent | 95% ethanol | + | ++ | ++ | ++ |
| Water | + | ++ | ++ | ++ | ||
| Tannins | Ferric chloride Test | 95% ethanol | + | + | + | + |
| Water | + | + | + | + | ||
| Anthocyanins | HCl test | 95% ethanol | + | + | + | + |
| Water | ++++ | + | ++ | +++ | ||
| Alkaloids | Wagner’s test | 95% ethanol | + | + | + | + |
| Water | + | + | + | + | ||
| Saponins | Frothing test and Olive oil test | 95% ethanol | ++ | ++ | + | + |
| Water | + | ++ | + | + | ||
| Terpenoids | Salkowski test | 95% ethanol | + | + | ++ | ++ |
| Water | + | + | + | + | ||
| Cardiac glycosides | Keller-Killians Test | 95% ethanol | - | - | - | - |
| Water | - | - | - | - | ||
| Steroids | Salkowski test | 95%ethanol | - | - | - | - |
| Water | - | - | - | - | ||
| Anthraquinone glycosides | Borntrager test | 95%ethanol | - | - | - | - |
| Water | - | - | - | - |
Note: Values are mean ± standard deviation of the six independent determinations.
(+) =indicate presence; (-) =indicate absence; (+) =midly positive; (++) =moderate positive, (+++,++++) =high positive.
Table 4: The phytochemical analysis of secondary compounds in banana blossom extracts of Musa sapientum Linn. (Musa ABB group, cv. Namwa)
Antioxidant activity of banana blossom
Total Phenolic Contents (TPC)
The Folin-Ciocalteu assay was used in the Total Phenolic Content (TPC) determination of different extracts as well as Gallic acid was used as a standard. Phenolic compounds are plant secondary metabolites that have redox properties to act as antioxidant activity. The hydroxyl groups are responsible for facilitating free radical scavenging and inactivating lipid free radical chains [29]. The results were derived from a calibration curve (y=0.0016x+0.0026, R2=0.9997) of Gallic acid (12.5-500 μg/mL) and expressed as milligrams of Gallic acid equivalents per gram of dry extract (mg GAE/g). Table 5 showed that the total phenolic content value of each part extracts of banana blossom (Musa sapientum) revealed in the range of 86.07 ± 1.03 to 168.88 ± 1.47 mg GAE/g (P<0.05). The contents of total phenolic compounds in water extracts of the bract, floret, young blossom, and mixed parts were 147.27 ± 1.69, 120.11 ± 1.93, 114.45 ± 1.64 and 143.18± 1.93 mg GAE/g, respectively, there was no significant difference in the total phenolic contents of water extracts of all parts of the banana blossom. The total phenolic contents in ethanol extracts of the bract, floret, young blossom, and mixed parts were 145.12 ± 1.82, 168.88 ± 1.47, 86.07 ± 1.03, and 135.16 ± 0.78 mg GAE/g, respectively there was no significant difference in the total phenolic contents of water extracts of all parts of the banana blossom. The total phenolic contents in ethanol extracts of the bract, floret, young blossom, and mixed parts were 145.12 ± 1.82, 168.88 ± 1.47, 86.07 ± 1.03, and 135.16 ± 0.78 mg GAE/g, respectively (P<0.05). The ethanol extract of floret showed the greatest total phenolic content (168.88 ± 1.47 mg GAE/g), followed by bract water extract (147.27 ± 1.69 mg GAE/g), bract ethanol extract (145.12 ± 1.82 mg GAE/g), floret water extract (120.11 ± 1.93 mg GAE/g), and young blossom water extract (114.45 ± 1.64 mg GAE/g), while the least was found in young blossom ethanol extract (86.07 ± 1.03 mg GAE/g) (P<0.05).
| Extracts | Total phenolic contents (mg GAE/g extract) | |||
|---|---|---|---|---|
| Bract | Floret | Young blossom | Mixed parts | |
| Water extract | 147.27 ± 1.69ab | 120.11 ± 1.93ab | 114.45 ± 1.64ab | 143.18 ± 1.93ab |
| 95% Ethanol extract |
145.12 ± 1.82ab | 168.88 ± 1.47a | 86.07 ± 1.03b | 135.16 ± 0.78ab |
Note: Values are mean ± standard deviation of the six independent determinations.
a-cThe letters indicate singnificant differences in extracted parts of banana blossom (P< 0.05).
Table 5: The total phenolic contents in banana blossom extracts of Musa sapientum Linn. (Musa ABB group, cv. Namwa)
Inhibition of lipid peroxidation
The percentage of lipid peroxidation inhibition by 95% ethanol extracts and water extracts were resulted that bract, floret, young blossom and mixed parts of Musa sapientum Linn. (Musa ABB group, cv. Namwa) were significant difference (P<0.05). The Ethanol extracts and water extracts of bract, floret, young Fiblossom, and mixed parts inhibited lipid peroxidation that lipid peroxidation was induced by ferrous sulfate in egg yolk homogenates at a concentration- dependent manner. All extracts showed concentration-dependent inhibition. However, when the concentration of extract decreases, it would reduce the inhibition of lipid peroxidation. The ethanol extracts of the bract, floret, young blossom, and mixed parts had less lipid peroxidation inhibitory activities than water extracts at any concentration (P<0.05). There was no significant difference in the inhibition of lipid peroxidation of water extracts of bract, floret, young blossom, and mixed parts at each level of concentration. The ethanol extracts of floret at 10 and 1 mg/mL concentrations showed greater lipid peroxidation inhibition than bract and young blossom (P<0.05) (Table 6 and Figure 2).
| Extracts | Concentrations (mg/mL) | Inhibition of lipid peroxidation (%) | |||
|---|---|---|---|---|---|
| Bract | Floret | Young blossom | Mixed parts | ||
| Water extract | 0.1 | 78.57 ± 0.69X | 76.75 ± 1.09X | 74.04 ± 1.02X | 76.19 ± 1.54X |
| 95% ethanol extract | 0.1 | 36.69 ± 0.04Y | 46.40 ± 1.39Y | 36.92 ± 1.01Y | 41.49 ± 1.70Y |
| Water extract | 1 | 86.84 ± 1.78A | 83.97 ± 1.38A | 83.93 ± 1.29A | 85.69 ± 1.78A |
| 95% ethanol extract | 1 | 44.41 ± 1.48C | 56.66 ± 1.48B | 39.84 ± 1.87C | 49.51 ± 1.05BC |
| Water extract | 10 | 97.33 ± 0.75a | 95.23 ± 1.03a | 94.61 ± 1.04a | 95.65 ± 2.57a |
| 95% ethanol extract | 10 | 55.07 ± 1.93c | 88.82 ± 1.17ab | 54.04 ± 1.58c | 74.85 ± 1.51b |
Note: Values are mean ± standard deviation of the six independent determinations.
a-c, A-C and X-Y The letters indicate singnificant differences in extracted parts of banana blossom and at each concentration groups (P< 0.05).
Table 6: Percentage inhibition of lipid peroxidation activity of banana blossom extracts of lim sapientum Linn. (Musa ABB group, cv. Namwa) at different concentration, using egg yolk homogenates as media.
(Figure 3A) showed the Total Phenolic Contents (TPC) of water extract of the bract, floret, young blossom, and mixed parts of Musa sapientum were not significant difference, while the ethanol extract of the floret contained the total phenolic contents of the greatest, followed by the bract water extract and the young blossom contained the least. According to the percentage of lipid peroxidation inhibition (Figure 3B), the result indicates that total phenolic content iscorrelated with inhibition of lipid peroxidation activities. (Figure 3)
Discussion
In this study, the banana blossom of Musa sapientum Linn. (Musa ABB group, cv. Namwa) consists of several parts such as bract, floret, and young blossom. The banana blossom of Musa sapientum contained 93.05% moisture that is quite related to the other study of same species in Thailand, 91.8-92.2 g/100 g [30], and other species for example (Baxijiao and Paradisiaca 89.42 and 90.58 g/100 g, respectively) from China [31], including Poovan and Monthan (90.1 and 90.23 g/100 g, respectively) from India [32]. All these blossoms contain high moisture levels, implying that they will have a short shelf life [31].
The yield percentage of water extract and ethanol extract of the banana blossom of Musa sapientum found that water extracts yielded greater than ethanol extracts. The result of this study is relating to the results of other studies in Thailand [6]. Thus, water extract of dry young blossom demonstrates the greatest yield, while the 95% ethanol extract of young blossom demonstrates the least.
The nutritional composition of its parts of Musa sapientum of Thailand contained crude protein varied from 7.19 to 20.09% DM, young blossom demonstrated the greatest meanwhile bract demonstrated the least. Mixed parts of blossom contained crude protein of 13.86% DM (1.31 g/100 g) that less than Paradisiaca flower 1.62 g/100 g and Baxijiao flower g/100 g, from China, [31], including Poovan flower 1.99 g/100 g and Monthan flower 1.43 g/100 g, from India, [32]. In addition, the banana blossom contained major amino acid compositions, for instance glycine, leucine, alanine, and aspartic acid, however, lysine was found at the least amount [31]. The different blossom parts of Musa sapientum contained crude fat content in the range of 3.08%-7.15% DM, especially the mix parts of blossom contained crude fat content of 4.56% DM (0.43 g/100 g), which was the same amount as Baxijiao flower 0.4 g/100 g and Poovan flower 0.43 g/100 g but less than Paradisiaca flower 0.6 g/100 g and Monthan flower 0.54 g/100 g [31,32]. However, the difference between protein and fat content is mainly due to different genotypes. Blossom parts of the Musa sapientum contained total ash content of 13.02–16.05%DM, and the mixed parts also contained the total ash of 14.35% DM (1.36 g/100 g), which is greater than the Baxijiao flower and Paradisiaca flower 1.19 g/100 g and 1.24 g/100 g, respectively [31]. Blossom parts of the Musa sapientum contained crude fiber of 19.58-28.79% DM. The bract contained the greatest, while the mixed parts contained crude fiber content of 23.78% DM (2.25 g/100 g). According to the research report of Baxijiao flower and Paradisiaca flower, which contained dietary fiber of 4.96 and 5.74 g/100g, respectively [31]. A greater content of fiber of banana blossom indicates that it can be consumed as dietary fiber supplements. Moreover, they contribute many health benefits in reducing ulcers, improving bowel movements, and maintaining gut health [33]. High levels of dietary fiber intake are associated with lower risk of Coronary Heart Disease (CHD), Peripheral Vascular Disease (PAD) and ischemic stroke [34-36].
The phytochemical of 95% ethanol extract and water extract of the bract, floret, young blossom and mixed parts of Musa sapientum showed the presence of the same compounds, such as flavonoids, phenolic compounds, anthocyanins, tannins, alkaloids, saponins, and terpenoids which are secondary metabolites compounds and impotent medicinal phytochemicals groups making biological activities. These results are also corresponded to a study of Rao et al., reported that tepal (was removed from the bracts) in all three extracts (methanolic, ethanolic and aqueous) of other species of Musa paradisiaca, which found the presence of phenolics, flavonoids, alkaloids, tannins and terpenoids, but saponins was not found [35]. However, Mahmood et al., [36] reported that the flower parts of M. paradisiac contained saponins, flavonoids, and tannins in 95% ethanol extract, but flavonoids and terpenoids were found only in the floret, but not found in the bract parts of the flower. Aqueous extract showed that there were only saponins and tannins, while petroleum ether extract showed alkaloids, glycosides, and steroids. This difference may be due to the differences of banana species, solvent, and methods [37].
The presence of flavonoids, anthocyanins, and tannins is responsible for free radical scavenging; however, anthocyanins are classified as flavonoids [38]. Flavonoids exhibits several biological activities, such as antioxidant, anti-inflammatory, antimicrobial, anticancer, and etc., Flavonoids and tannins are phenolic compounds, which are a major group of compounds that act as primary antioxidants or free radical scavengers [39]. Flavonoids are the largest group of naturally occurring phenolic compounds, while tannin compounds are used for health benefits as an antioxidant, anti-allergic, anti- helminthic astringent and hemostatic qualities. These compounds are able to relieve wounds heal faster and reducemucus membrane inflammation [40,41]. Phenolic compounds from medicinal plants are the largest group of phytochemicals such as flavonoids, tannins, and saponins that possess strong antioxidant activity and are able to protectthe cells against oxidative damage from free radicals inducing oxidative stress [42-45]. Saponins boost body immunity, reduce cholesterol, and prevent the growth of cancer cells [46]. Phenolics possess biological activities such asantioxidant, antimutagenic, anti-carcinogenic, and even the ability to modify gene expression [47]. Saponins are treated for expectorants that are crucial in the treatment of inflammation of the upper respiratory tract, anti-fungal, and anti-diabetic activities [48]. Terpenoids possess anti-inflammatory, antimalarial, antiviral, antibacterial, antitumor and inhibition of cholesterol synthesis [49]. Alkaloids are the largest group of secondary chemical constituents made largelyof ammonia compounds. The solutions of alkaloids are intensely bitter that they are widely used as pharmaceuticals, stimulants, narcotics, and poisons [50,51]. In this study, all the parts of banana blossom extracts of Musa sapientum Linn. (Musa ABB group, cv. Numwa) revealed the presence of flavonoids, phenolic compounds, anthocyanins, tannins,alkaloids, saponins, terpenoids. Therefore, banana blossoms of Musa sapientum Linn. (Musa ABB group, cv. Numwa) exhibits potential antioxidant activities. Antioxidants are reactive chemical compounds from plant materials that act as free radical scavenging to protect human, animal, and plant cells from various diseases [52].
Phenolic compounds are quite important constituents of plant because they exhibit antioxidant activity by preventing the decomposition of hydroperoxides into free radicals and also by inactivating lipid free radicals. The results of this study found that banana blossom and its parts of Musa sapientum contained a great total phenolic content that demonstrates antioxidant properties. The ethanol extract of floret showed the greatest total phenolic content, while young blossom ethanol extract was found at the least amount. However, the water extracts of bract, floret and young blossom, includingethanol extracts of bract and young blossom showed similar values of total phenolic content. In addition, the both water extract and ethanol extract of mixed parts of blossom reveal similar total phenolic content. The banana blossom of Musasapientum showed a greater total phenolic content of ethanol extract (135.16 ± 0.78 mg GAE/g) and water extract (143.18 &p 1.93 mg GAE/g) than banana flowers of Baxiijiao and Paradisiaca (10.02 ± 0.03 and 6.58 ± 0.03mg GAE/g ethanol extract) from the China. This can be concluded that the banana blossoms of Musa sapientum are quite good sources of total phenolic contents according to the phenolic compounds reported the main phytochemicals, as shown in Table 4. Thus, total phenolic contents might be considered as a correlation with its activities.
Lipid peroxidation is a process under oxidants like the free radicals or non-radical species that remove off hydrogen and add oxygen up carbon-carbon double bond(s) of lipids, especially Polyunsaturated Fatty Acids (PUFAs). Therefore, this results to create lipid peroxyl radicals, and hydroperoxides. Free radical induced-lipid peroxidation has been associated with a variety of disease processes and pathological, such as diabetes, inflammation, and cancer. Meanwhile, lipid peroxidation activity has been evaluated for antioxidant activity and also inhibition of lipid peroxidation is considered as the most important index of antioxidant potential.
The Banana blossom of Musa sapientum Linn. (Musa ABB group, cv. Namwa) demonstrated high potential in terms of lipid peroxidation inhibition. All parts of the banana blossom showed concentration-dependent lipid peroxidation inhibition. The inhibition percentage of lipid peroxidation by 95% ethanol and water extracts of the bract, floret, young blossom, and mixed parts were statistically significant differences (P<0.05). All parts of banana blossom extracts by water showed a greater percentage of inhibition than all the parts of banana blossom extracts by 95% ethanol at all concentration levels (P<0.05). Whereas, the inhibition of lipid peroxidation in each concentration of water extract of the bract, floret, young blossom, and mixed parts were not significant. However, the inhibition percentage of lipid peroxidation of water extract of the bract showed the greatest tendency, while the 95% ethanol extracts of the floret showed the greatest. All parts of banana blossom could prevent the initiation of lipid peroxidation by chelating or reducing the iron or by scavenging the free radical produced within the propagation phase of lipid peroxidation.
Aside from this, the study of Lizcano et al., [52], resulted polyphenols from medicinal plants inhibited lipid oxidation byacting as chain-breaking peroxyl-radical scavengers. To support this study found, the great total phenolic contents of water extracts and ethanol extracts of all parts of the blossom, especially, the bract water extract and floret ethanol extract. According to the percentage of lipid peroxidation inhibition, this result indicates that total phenolic content is correlatedto inhibition of lipid peroxidation activity.
In this study, all parts of the banana blossom of Musa sapientum Linn. (Musa ABB group, cv. Namwa) contained high nutrients, mainly fiber, carbohydrate, protein, ash, calcium and phosphorus. Thus, it’s a source of various phytoconstituents such as phenolic compounds, flavonoids, anthocyanins, tannins, alkaloids, saponins, and terpenoids. Moreover, it is also found high levels of total phenolic content, and high lipid peroxidation inhibition. Therefore, banana blossom (and various parts of it) possess the potential as a source of natural antioxidants, which is reducing the breakdown of fatty acids from the peroxidation reaction and may be inhibiting or preventing the deleterious consequences of oxidative stress, for their benefits of natural medicine and therapeutic food/feed including oxidative stress reducing. The results from this study can be suggested that water decoction of banana blossom could be used to prevent oxidative stress better than blossom 95% ethanol extract.
Conclusion
All parts of banana blossom of Musa sapientum Linn. (Musa ABB group, cv. Namwa) show quite valuables of high nutritional features, such as fiber, protein, carbohydrate, calcium and phosphorus, especially, rich fiber can be consumed as dietary fiber supplements, provided various health benefits in reducing ulcers, improving bowel movements, and maintaining gut health. In addition, banana blossom is a source of various natural antioxidant phytoconstituents, for instance phenolic compounds, flavonoids, anthocyanins, tannins, alkaloids, saponins, and terpenoids that it’s the secondary metabolites, contain numerous natural products including it has interesting pharmacological properties. Banana blossom also possesses high total phenolic content and high lipid peroxidation inhibition. Moreover, total phenolic content is correlated with inhibition of lipid peroxidation activities.
For the reason that banana blossom (and various parts of it) possess the potential as a source of natural antioxidants which will be able to decrease lipid peroxidation by reducing oxidative stress for their benefits as a source of natural medicine and therapeutic food/feed including oxidative stress reduction that they contribute to the relief and healing of human and animal ailments. Therefore, banana blossom and its various parts of blossom possess the potential benefits as the dietary supplementation and antioxidant for human and/or animal health that this study investigated and proved valuable compositions of banana blossom as well. For the next study, the researcher (author) will do is the implementation of these banana blossom for using as a dietary supplementation in animal feed, antioxidant, and natural medicine in order to confirm nutritional and pharmaceutical values of banana blossom.
References
- Martin G, Carreel F, Coriton O, et al. Mol Biol Evol. 2017;34(9): 2140-2152.
[Crossref] [Google Scholar] [PubMed]
- Aiemcharoen P. (Doctoral dissertation, Prince of Songkla University). 2022.
- Robinson JC. Wallingford, UK: CAB International. 1996; 238 pp.
- Zozimo RO, Ratanasut K, Boonsrangsom T, et al. Int J Biosci. 2018;12(4):172-180.
- Khongkhon JA. Faculty of Medicine Thammasat University, Thailand, 2016.
- Singh S. Int J curr Res. 2017; 9(01):44516-44519.
- Sidhu JS, Zafar TA. Food Qual Saf. 2018;2(4):183-188.
- Lau BF, Kong KW, Leong KH, et al. Trends Food Sci Technol. 2020;97:14-28.
- Pushpaveni C, Visagaperumal D, Vineeth C. World J Pharm Res. 2019;8(11):440-450.
[Crossref]
- Begum YA, Deka SC. J Food Sci Technol. 2019;56(12):5298-5308.
[Crossref] [Google Scholar] [PubMed]
- Bhaskar JJ, Mahadevamma S, Nandini DC, et al. J Agric Food Chem. 2012;60(1):427-432.
[Crossref] [Google Scholar] [PubMed]
- Sharmila TD, Puraikalan YD. Int J Sci Res. 2015;4(4):1409-1411.
- Loganayaki N, Rajendrakumaran D, Manian S. Food Sci Biotechnol. 2010;19(5):1251-1258.
[Crossref] [Google Scholar] [PubMed]
- Yen GC, Duh PD, Tsai HL. Food Chem. 2002;79(3):307-313.
- Suffi NS, Mohamed E, Camalxaman SN, et al. Healthscope. 2021; 4(1): 113-118.
- Jawla S, Kumar Y, Khan MS. Asian Pac J Trop Biomed. 2012; 2(2): S914-S918.
- Sheng Z, Dai H, Pan S, et al. Mol. 2014; 19(7): 10563-10573.
[Crossref][Google Scholar] [PubMed]
- Ramu R, Shirahatti PS, Zameer F, et al. J Sci Food Agric. 2015; 95(1): 165-173.
[Crossref][Google Scholar] [PubMed]
- AOAC. Offic Method Analy. 1990.
- Steenfeldt S, Pettersson D. J Anim Feed Sci. 2001; 10(1): 143-158.
- Shaikh JR, Patil M. Int J Chem Stud. 2020; 8(2): 603-608.
- Savithramma N, Rao ML, Suhrulatha D. Middle-East J Sci Res. 2011; 8(3): 579-584.
- Edeoga HO, Okwu DE, Mbaebie BO. African J Biotechnol. 2005; 4(7): 685-688.
- Seladji M, Bekhechi C, Bendimerad N. Int J Pharm Sci Rev Res. 2014;26(1):40.
- Apak R, Capanoglu E, Shahidi F, et al. John Wiley Sons Ltd. 2017; 1: 107-115.
- Upadhyay R, Chaurasia JK, Tiwari KN, et al. Sci World J. 2014.
[Crossref][Google Scholar] [PubMed]
- Soobrattee MA, Neergheen VS, Luximon-Ramma A, et al. Mutat Res. 2005; 579(1-2): 200-213.
[Crossref][Google Scholar] [PubMed]
- Somsub W, Kongkachuichai R, Sungpuag P, et al. J Food Compos Anal. 2008; 21(2): 187-197.
- Sheng ZW, Ma WH, Jin ZQ, et al. Afr J Biotechnol. 2010;9(25):3888-3895.
- Arya Krishnan S, Sinija VR. Int J Agric Sci Food Technol. 2016.
- Anderson JW, Baird P, Davis RH, et al. Nutr Rev. 2009;67(4):188-205.
- Liu S, Stampfer MJ, Hu FB, et al. Am J Clin Nutr. 1999;70(3):412-419.
- Merchant AT, Hu FB, Spiegelman D, et al. J Nutr. 2003;133(11):3658-3663.
- Liu S, Manson JE, Stampfer MJ, et al. JAMA. 2000;284(12):1534-1540.
- Rao M, Abdurrazak M, Mohd KS. Malaysian J Anal Sci. 2016;20(5):1181-1190.
- Mahmood A, Ngah N, Omar MN. Eur J Sci Res. 2011;66(2):311-318.
- Sharma K, Kumar V, Kaur J, et al. Toxin Rev. 2021;40(4):432-444.
- Ajuru MG, Williams LF, Ajuru G. Int J Food Sci Nutr. 2017;5(5):198-205.
- Sulaiman CT, Balachandran I. Indian J Pharm Sci. 2012;74(3):258.
- Shahidi F, Janitha PK, Wanasundara PD. Crit Rev Food Sci Nutr. 1992;32(1):67-103.
- Kähkönen MP, Hopia AI, Vuorela HJ, et al. J Agric Food Chem. 1999;47(10):3954-3962.
- Ozsoy N, Can A, Yanardag R, et al. Food Chem. 2008;110(3):571-583.
- Nann K; Swe N. Universities Res J. 2012; 5(1): 1-5.
- Ribarova F, Atanassova M, Marinova D, et al. JU Chem. Metal. 2005; 40(3): 255-60.
- Mahato SB, Sen S. Advances in triterpenoid research, 1990-1994. Phytochemistry. 1997;44(7):1185-1236.
[Crossref] [Google Scholar] [PubMed]
- Madziga HA, Sanni S, Sandabe UK. J Am Sci. 2010;6(11):510-514.
- Lai LS, Chou ST, Chao WW. J Agric Food Chem. 2001;49(2):963-968.
[Crossref] [Google Scholar] [PubMed]
- Maisuthisakul P, Suttajit M, Pongsawatmanit R. Food Chem. 2007;100(4):1409-1418.
- Sheng ZW, Ma WH, Gao JH, et al. Afr J Biotechnol. 2011;10(21):4470-4477.
- Yin H, Xu L, Porter NA. Chem Rev. 2011;111(10):5944-5972.
[Crossref] [Google Scholar] [PubMed]
- Baynes JW. Diabetes. 1991;40(4):405-412.
[Crossref] [Google Scholar] [PubMed]
- Lizcano LJ, Viloria-Bernal M, Vicente F, et al. Int J Mol Sci. 2012;13(5):5454-5467.
[Crossref] [Google Scholar] [PubMed]