Short communication: 2021 Vol: 13 Issue: 3
Nodulisporium terra: A New Fungal Species Explored from Soil of Paddy Field, Raman Kari, Alappuzha District, Kerala, India
Shigi Joseph* and Neeta N. Nair
Post graduate and Research Department of Botany, Mar Thoma College, Thiruvalla-689103, Kerala, India
Abstract
In the present investigation, a new fungal species, Nodulisporium terra was isolated and identified from the soil samples collected from Paddy field of Raman Kari, Kerala state of India. The current study is the first study ever done to report the said species. The above new species were reviewed and compared with the previously known species, and the differentiating characteristics were reviewed and considered to represent a new species.
Keywords
Ascomycota; Soil microfungi; Xylariaceae; Fungal diversity; Kuttanadu
Introduction
The genus Nodulisporium [1] was erected from Germany based on type species Nodulisporium ochraceum Preuss. The type species reportedly is not dematiaceous. The generic concept of Nodulisporium has been well summarized and illustrated [2]. Thus the genus Nodulisporium contains both dematiaceous and non dematiaceous members and occur in nature worldwide, through being conidial anamorphs of certain wood decay ascomycetes like Hypoxylon, Xylaria, Daldinia, Entonaema, and Biscogniataia [3-8].
Conidial genera that can be confused with Nodulisporium include Calcarisporium, Geniculosporium, Hansfordia, Phaeoisaria, Rhinocladiella, Sporothrix, and Virgaria [2,9-11]. The Nodulisporium anamorph grows in conjunction with the developing stromata briefly during favourable conditions and, in few cases, is able to grow independently on various organic matter with Nodulisporium like conidiophore branching pattern [12] whereby successive dichotomous or trichotomously branched conidiophores gives rise to multiple levels of terminal branches and all bearing 1–3 (rarely more) conidiogenous cell.
Experimental Section
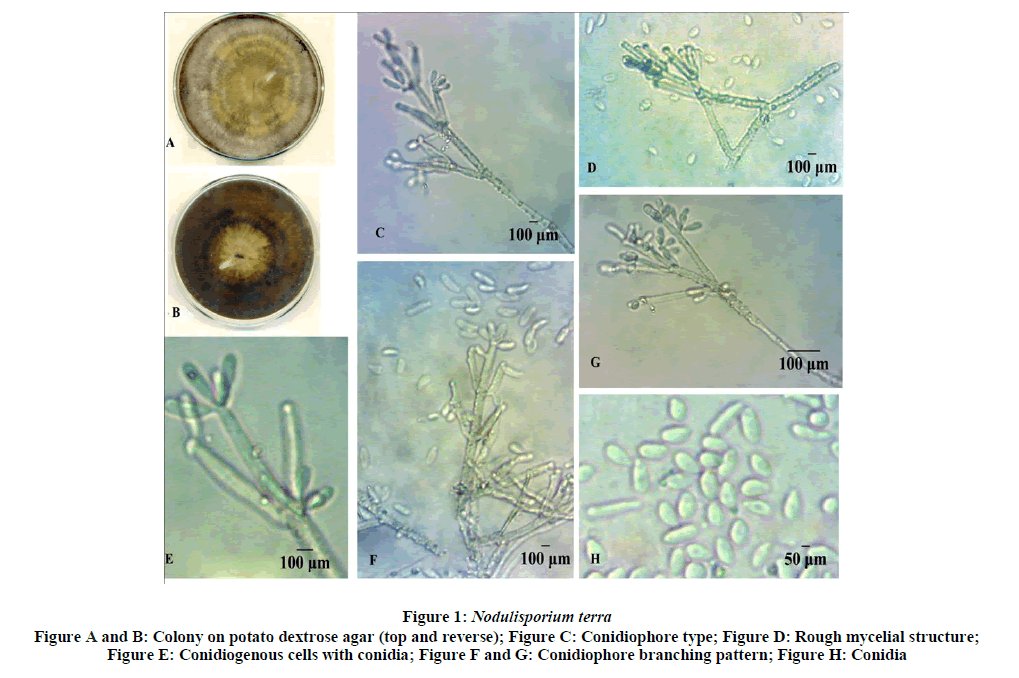
Soil samples were collected from paddy field of Ramankari ,Kuttanad Alapuzha district, Kerala at randomly from a depth of 0-15 cm and mixed together to get one composite soil sample. The soil dilution method on Potato Dextrose Agar was used as isolation technique (Figure 1) [13].
Results
Mycobank MB 837464
Etymology: The specific epithet is named after it isolated from soil
Mycelium superficial to partially immersed, hyphae colour, rough to denticulate, loosely septate 1.5-2.5 μm wide. Colonies on PDA growing up to 7.8 mm in diameter after 10 days at 250 ± C, finely floccose, zonate, slightly cream to yellowish colored with white margin later turning with greyish-brown surface, yellowish pigment diffuse in culture medium. Stromata and any other odor were not observed. Conidiophores were mononematous, macronematous, branched usually at terminal end, flexuous, olivaceous to pale brown, septate, smooth at basal portion or denticulate up to terminal end mostly 600-750 μm long × 6.5 μm wide towards the base,5 um wide towards the apex. Conidiogenous cells develop on the branches of conidiophores, polyblastic, integrated or terminal or discrete, solitary or arranged penicillately, sympodial, cylindrical up to 30 μm long × 6 μm wide, denticulate; denticles short, fragile, sometimes swollen in the middle otherwise long cylindrical bearing conidia at the swollen tip or some time along the side denticles , holblastic conidiogensis. Conidia hyaline, nonseptate, smooth, guttulate with 1-2 oil globules, variably shaped, ellipsoidal to fusoid or obovoid, pyriform, and cylindrical, 10-12 μm long × 5.5 μm broad, with small flat truncate basal scar of 2-3 μm diam, Soil samples from Paddy field of Ramankari, Alappuzha district, Kerala state of India. Collected by Shigi Joseph July, 2019, NCFT, No 9811 .20
Discussion and Conclusion
The new species was compared with species of Hughes (1958) who erected seven comb novo based on genus Botrytis, Haplaria, Sporotrichum, Dematium and Trichosporum namely Nodulisporium atroviride, Nodulisporium affine and Nodulisporium ellisii based on (=Botrytis atroviridis, Botrytis affinis, Botrytis ellisii) respectively in addition to Nodulisporium corticioides of (=Haplaria corticioides), Nodulisporium fulvum of (=Sporotrichum fulvum), Nodulisporium episphaerium of (=Dematium episphaericum), Nodulisporium tabacinum of (=Trichosporum tabacinum).Thereafter, Deighton, made four comb novo based on genus Isaria and Verticillium species namely Nodulisporium radians, Nodulisporium acervatum, Nodulisporium umbrinum based on Isaria radians, Isaria acervata, Isaria umbrina respectively while, Nodulisporium puniceum of Verticillium puniceum and Nodulisporium sylviforme which is exclusively based on mononematous structure and typical Nodulisporium type branching pattern of conidiophores.
Eleven Nodulisporium species cited above were reviewed for their taxonomical characters with regards to colony growth, conidiophores, conidiogenous cell and conidia formation in the light of their shape, size; color, septation, formation to justify the new taxon. Non amongst the above cited species do not found taxonomically closer in above cites characters to the new taxon of Nodulisporium terra. However, amongst the above the Nodulisporium sylviforme which have particular character of mononematous conidiophore and typical Nodulisporium type branching pattern were specifically compared with new proposed species while other remaining species excluded due to presence of having different stages like Botrytis, Haplaria, Sporotrichum, Dematium Trichosporum, Isaria, and Verticillium type. In contrast to the new species have rough to denticulate mycelial structure, longer conidiophores, smooth, larger conidiogenous cells, longer conidia with enlarged to cylindrical in shape with distinct oil globules , thus Nodulisporium terra sp.nov is being proposed [14,15].
Acknowledgment
The authors are highly thankful to the services rendered for confirming above new taxon by Dr. P.N.Chowdhry, Principal Mycologist, National Centre of Fungal Taxonomy (www.ncft.in), New Delhi and Dr. Priyanka Chouhan Jr. Mycologist for assisting in review of literature to justify above new species.
References
- GL Barron. The Genera of hyphomycetes from soil, Robert E. Krieger Publishing Co. Huntington, New York, 1968; 364.
- BE Callan; JD Rogers. Canadian Journal of Botany. 1986; 64, 842-847.
- FC Deighton. Transactions of British Mycological Society. 1985, 85 (3), 391-395
- MB Ellis. Dematiaceous hyphomycetes. Commonwealth Mycological Institute, Kew, Surrey, England, 1971; 608
- GN Greenhalgh; CGC Chesters. Trans. Br. Mycol. Soc. 1968; 51, 57-82.
- SJ Hughes. Canadian Journal of Botany. 1958, 36(6), 727-836
- YM Ju; JD Rogers. A revision of the genus Hypoxylon. American Phytopathological Society, St. Paul. USA, 1996; 365.
- SC Jong; JD Rogers. Illustration of conidial states of some Hypoxylon species. Washington Agricultural Experiment Station Technical Bulletin. 1972; 71, 1-51.
- MR Ginnis; WA Schell. The genus Fonsecaea and its relationship to the genera Cladosporium, Phialophora, Ramichloridium, and Rhinocladiella. PAHO scientific publication Pan American Health Organization, Washington. D.C. 1980; 215-224
- JH Miller. A monograph of the world species of Hypoxylon. University of Georgia Press, Athens, Georgia. 1961; 158
- C Preuss. Germaniam crescentium collectionem. 1849, 1272.
- JD Rogers. Mycologia. 1966, 8, 459-465
- JD Rogers. Mycotaxon. 1982, 15, 500-506.
- G Smith. Transactions of British Mycological Society. 1962, 45, 387-94.
- SA Waksman. Journal of Bacteriology. 1922, 7, 303?309