Original Articles: 2021 Vol: 13 Issue: 10
Improvement of Solubility and Dissolution by Liqui-Solid Compact
Abstract
Liqui-solid technique is a new technique for the drugs which has solubility problem. This technique proved to increase dissolution of poor water soluble drugs. In this technique liquid medications such as solutions or suspensions of water insoluble drugs in suitable non-volatile liquid vehicles can be changed into suitably flowing and compressible powders via blending with elected powder excipients. In these, drug is held in solution around and excipient powder in surrounding, so drug is present in solubilised form, which increases dissolution.
Keywords
Liqui-solid compacts; Liquid medication; Low solubility drugs
Introduction
The solubility of a lot of active pharmaceutical ingredients is one of the technical challenges in formulating as suitable dosage form for efficient drug delivery. Most of the hydrophobic drugs termed as sparingly soluble, slightly soluble and very slightly soluble, undergo very poor dissolution in the gastro intestinal tract, leading to unpredictable and incomplete absorption. For these drugs, the dissolution process is the rate-controlling step, which determines the rate and degree of its absorption. Nearly 40%-50% of newly developed and orally administered drugs exhibit solubility problem in aqueous media due to its high lipophilicity, which directly reflects in the difficulty in formulation development of those drugs. Even though oral route is the most preferred route for drug administration due to its fulfilment of necessary strategies for drug development and patient acceptance, the poorly soluble drugs generally exhibit slow dissolution rates and incomplete bioavailability due to poor wettability in the Gastro Intestinal Tract (GIT).
The drugs belonging to the Biopharmaceutical Classification System (BCS) class II and IV dissolve slowly, poorly or irregularly, which results in incomplete release of the drug from the dosage form, increase in the dose, large inter and intra-subject variation in blood drug concentrations under fed and fasted conditions ultimately leading to poor bioavailability [1]. Over past few years, various formulation techniques have been developed, to improve the solubility and dissolution of poorly soluble substances, with different degrees of success. There are multiple methods which have been used for past many years, to enhance the dissolution characteristics of water insoluble drugs which include micronization, lyophilization, solid dispersion etc.
Out of which the recent research focus on liquid-solid compact technique as one of the successful tool to achieve the goal. Liqui-solid compacts are acceptably flowing and compressible powder forms of liquid medications. The liquid medication is the water insoluble drugs carried in suitable non-volatile solvents. This liquid medication is converted into a free flowing powder by addition of suitable excipients. The concentrations of the carriers, coating materials, disintegrants, lubricants and glidants are optimized to get a non-sticky easily compressible blend [2]. This technology ensures the promotion of dissolution rate of poorly water soluble drugs since the drugs are completely solubilised in the suitable solvents before converting it into free flowing mass.
The drug in the solid dosage form is held within the powder substrate in a solution or in a solubilized, almost molecular dispersion level, which is the main reason for its significant change in the wetting properties and effective surface area. The drug available for dissolution is increased and hence show enhanced drug release characteristics and improved oral bioavaibility [3]. The carrier and coating powder material can retain only certain amounts of liquid while maintaining acceptable flow and compression properties depending on the excipients ratio. The powder excipients ratio R is the fraction of weight of carrier (Q) and the coating material (q) present in the formulation R=Q/q. The maximum liquid load on the carrier material is termed as the liquid load factor.
Lf is defined as the weight ratio of the liquid medication (w) and carrier powder (Q) in the system Lf=w/Q, which must be posed by an acceptable flowing and compressible preparation. The φ-value of a powder is the maximum amount of a given non-volatile liquid that can be holed inside its bulk (w/w) while maintaining the reasonable flow. The ψ-number of a powder is the maximum amount of liquid that can be retained by a powder within its bulk (w/w) while maintaining acceptable compactability, so that while making cylindrical compacts with enough strength ‘liquid-squeezing-out’ phenomena does not occur. The φ-value and the ?-number of powder may be determined using the procedure Liquid-Solid Flowability Test (LSF) and Liquid-Solid Compressibility (LSC) test, respectively [4-6].
The compaction techniques can be proceeded via direct compression method and slugging method. The formulation of liquid-solid compacts has several merits which include simplicity; low cost and capability of industrial scale up production. Diazepam is a potent anti-epileptic and anti-anxiety drug with narrow therapeutic index. Its poor solubility in water slows down the dissolution, leading to restricted absorption. The present work emphasis on the development of liquid-solid compact formulation for this deserving drug candidate to enhance its solubility and dissolution, thereby increase its absorption in the GIT. The dissolution properties of a drug and its release from a dosage form have a basic impact on its bioavailability [7]. Solving solubility problems is a major challenge for the pharmaceutical industry with developments of new pharmaceutical products, since nearly half of the active substances being identified through the new paradigm in high through put screening are either insoluble or poorly soluble in water. The rate of dissolution of a drug is a function of its intrinsic solubility and its particle size. Studies with poorly soluble drugs have demonstrated that particle size reduction to the sub-micron range can lead to an increase in dissolution rate and higher bioavailability.
Poorly water soluble drugs belong to BCS class II and class IV 7 group of compounds. In the process of absorption of drug from oral route; dissolution is the rate limiting step for lipophilic drugs; therefore it is necessary to enhance dissolution of these drugs to ensure maximum therapeutic utility of these drugs [8]. Dissolution is a process by which a solid substance goes into solution. The extents to which the dissolution proceeds, under a given set of conditions are referred to as the solubility of the substance in the solvent i.e. rate of solution (dissolution) and amount that can be dissolved (solubility) are not same. The dissolution rate of a drug is directly proportional to its solubility as per Noyes-Whitney equation and therefore solubility of a drug substance is a major factor that determines its dissolution rate and hence its absorption and bioavailability eventually.
The various properties of drug that affect drug dissolution and its rate includes solubility, particle size, polymorphism, salt form, complexation, wettability and can be targeted to enhance dissolution of poorly water soluble drugs. Micronization is the most common method to increase the drug surface area. But, in practice, the effect of micronization is often disappointing, especially when the drugs are encapsulated or tableted. The most promising method for promoting dissolution is the formation of liquid-solid tablets. Formulating liquid medications into solid compacts has been the object of many studies. On producing solid solutions and liquidsolids based on the concept of blending liquid medications with selected powder excipients to produce free flowing, readily compressible powders [9-11].
A liquid-solid system refers to formulations formed by conversion of liquid drugs, drug suspensions or drug solution in non-volatile solvents, into dry, non-adherent, free-flowing and compressible powder mixtures by blending the suspension or solution with selected carriers and coating materials. Various grades of cellulose, starch lactose, sorbitol may be used as the carrier; whereas very fine particle size silica powder may be used as the coating material. Sustained release dosage form is mainly designed for maintaining therapeutic blood or tissue levels of the drug for extended period of time with minimized local or systemic adverse effects. Economy and greater patient compliance are other advantages. In recent years, clinical studies on Tramadol Hydrochloride have demonstrated that this drug is an effective agent for moderate to severe chronic pain. The half-life of the drug is about 5.5 hours and the usual oral dosage regimen is 50 mg to 100 mg every 4 to 6 hours with a maximum dosage of 400 mg/day. To reduce frequent administration of dosage form and to improve patient compliance, a sustainedrelease formulation tramadol is desirable. The drug is freely water soluble and hence judicious selection of release retarding excipients is necessary to achieve a constant in vivo input rate of the drug. Various approaches have been used by researchers to sustain drug release in the form of tablets [12].
Literature Review
Historical development
Liqui-solid system is novel technique developed by Spireas. “Liqui-solid systems” involves conversion of liquid lipophilic drugs or water insoluble solid drugs dissolved in non-volatile solvent and this liquid medication can be converted into free-flowing, non adherent, dry looking, and readily compressible powders with use of carrier and coating materials [13]. In case of water soluble drugs, the sustained release can be obtained. The term liquid-solid compacts as described by Spireas, et al. indicates that immediate or sustained release tablets or capsules that are prepared using the technique of “liquid-solid systems” combined with inclusion of appropriate adjuvants required for tableting or encapsulation such as lubricants and for rapid or sustained release action, such as disintegrants or binders, respectively. Low cost, simple formulation technique and capability of industrial production serve to be advantages of this technique [10,14].
Historically, liquid-solid compacts are descendants of ‘powdered solutions’, an older technique which was based on the conversion of a solution of a drug in a non-volatile solvent into a dry-looking, no adherent powder by mainly adsorbing the liquid onto silica’s of large specific surfaces. Such preparations, however, have been investigated for their dissolution profiles while being in a powder dispersion form and not as compressed entities, simply because they could not be compressed into tablets. In later studies on powdered solutions, compression enhancers such as microcrystalline cellulose were added in such dispersions in order to increase the compressibility of the systems. In these studies, however, large quantities of silica’s were still being used, and the flow and compression properties of the products were never validated and standardized to industrial specifications and requirements. Specifically, when such modified powdered solutions were compressed into tablets, they presented significant ‘liquid squeezing out’ phenomena and unacceptably soft tablets, thereby hampering the industrial application of such systems [15].
Liqui-solid compacts, on the other hand, are acceptably flowing and compressible powdered forms of liquid medications, and have industrial application. In addition, the term ‘liquid medication’ does not\only imply drug solutions, as in powdered solutions, but also drug suspensions, emulsions, or liquid oily drugs. Therefore, in contrast to ‘powdered solutions’, the term ‘liqui-solid compacts’ is more general and it may encompass four different formulation systems namely,
• Powdered drug solutions
• Powdered drug suspensions
• Powdered drug emulsions
• Powdered liquid drugs
Furthermore, the earlier term ‘powdered solutions’ seems to be inadequate even in describing the original systems, since it has not been proven that the drug remains in solution in the liquid vehicle after its deposition on the extremely large powder surfaces of silica’s used [16].
Advantages of liquid-solid compact
• A great number of slightly and very slightly water-soluble and practically water-insoluble liquid and solid drugs such as Digitoxin, Prednisolone and Hydrocortisone etc. can be formulated into liquid-solid systems using the new formulation-mathematical model [17].
• Better availability of an orally administered water-insoluble drug is achieved when the drug is in solution form.
• Though the drug is in a tableted or encapsulated dosage form it is held in a solubilized liquid state, which consequently contributes to increased drug wetting properties, thereby enhancing drug dissolution.
• Production cost of liquid-solid systems is lower than that of soft gelatine capsules.
Advantage of liquid-solid systems, particularly for powdered liquid drugs, during dissolution of a liquid-solid tablet, after the disintegration process is completed, the drug solution or liquid drug, carried on the suspended and thoroughly agitated primary particles, is dispersed throughout the volume of the dissolution medium; such a phenomenon does not extensively occur during the dissolution process of soft gelatine capsule preparations [18]. Therefore, since more drug surface is exposed to the dissolving medium, liquid-solid systems exhibit enhanced drug release.
• Optimized rapid-release liquid-solid tablets or capsules of water-insoluble drugs exhibit enhanced in vitro and in vivo drug release as compared to their commercial counterparts.
• Optimized sustained-release liquid-solid tablets or capsules of water-insoluble drugs exhibit surprisingly constant dissolution rates (zero-order release) comparable only to expensive commercial preparations that combine osmotic pump technology and laser-drilled tablets.
Disadvantages of liquid-solid system
• The liquid-solid systems have low drug loading capacities and they require high solubility of drug in nonvolatile liquid vehicles.
• It requires more efficient excipients which have higher adsorption capacities which provide faster drug release with a smaller tablet size to improve liquid-solid formulations.
• To maintain acceptable flow ability and compatibility for liquid-solid powder formulation high levels of carrier and coating materials are require and that in turn will increases the weight of each tablet above 1 gm which is very difficult to swallow.
Need of liqui-solid system
The oral route remains the preferred route of drug administration due to its convenience, good patient compliance and low medicine production costs. In order for a drug to be absorbed into the systemic circulation following oral administration, the drug must be dissolved in the gastric fluids. Thus, one of the major challenges to drug development today is poor solubility, as an estimated 40% of all newly developed drugs are poorly soluble or insoluble in water [19]. In addition, up to 50% of orally administered drug compounds suffer from formulation problems related to their low solubility and high lipophilicity. Bioavailability of poorly water soluble hydrophobic drugs (class II in bio pharmaceutics classification system) is limited by their solubility and dissolution rate. The dissolution rate of these drugs can be improved by decreasing particle size, decreasing crystallinity, and/ or increasing the surface area. Several studies have been carried out to increase the dissolution rate of drugs by decreasing the particle size, by creating nanoparticles and micro-particles [20].
However, the fine drug particles have high tendency to agglomerate due to Vander-Waals attraction or hydrophobicity, which both result in a decrease in surface area over time. Another way of increasing the dissolution rate is adsorption of the drug onto a high-surface area carrier. In this technique, the drug is dissolved in an organic solvent followed by soaking of the solution by a high-surface-area carrier such as silica. Here, agglomeration of the drug particles is prevented due to the binding of drug to the carrier [21]. However, due to the presence of the residual solvent in the drug formulation, it is disadvantageous to use toxic solvents. To overcome the problem, the technique of ‘liqui-solid compacts’ is a new and promising approach towards dissolution enhancement. Liqui-solid compacts possess acceptable flowability and compressibility properties. They are prepared by simple blending with selected powder excipients referred to as the carriers and the coating materials. Many grades of cellulose, starch, lactose, etc. can be used as carriers, whereas silica’s of very fine particle size can be used as coating materials. In such systems, the drug existed in a molecular state of subdivision and systems were free flowing, onadherent, dry looking powders [22]. This technique was successfully applied for low dose water-insoluble drugs. Due to significantly increased wetting properties and surface area of drug available for dissolution, liquid-solid compacts of water insoluble substances may be expected to display enhanced drug release characteristics and, consequently, improved oral bioavailability. Since dissolution of a non polar drug is often the rate limiting step in gastro-intestinal absorption, better bioavailability of an orally administered water-insoluble drug is achieved when the drug is already in solution, thereby displaying enhanced dissolution rates. The technique of liquid-solid compacts has been successfully employed to improve the in vitro release of poorly water soluble drugs such as Carbamazepine, Famotidine, Piroxicam, Indomethacin, Hydrocortisone, Naproxen and Prednisolone [23].
Methodology
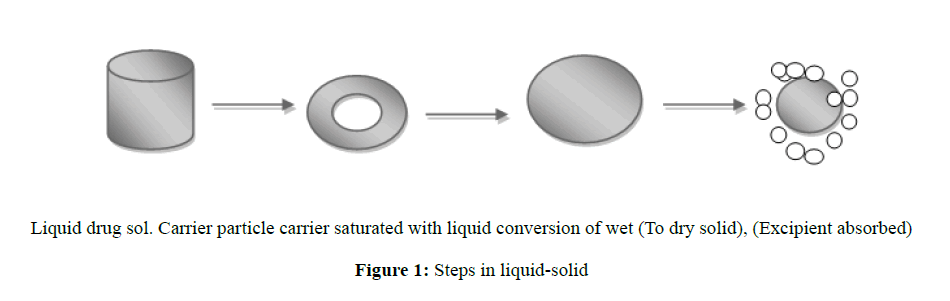
When the drug dissolved in the liquid vehicle is incorporated into a carrier material which has a porous surface and closely matted fibersin its interior as cellulose, both absorption and adsorption take place i.e. the liquid initially absorbed in the interior of the particles is captured by its internal structure, and after the saturation of this process, adsorption of the liquid onto the internal and external surfaces of the porous carrier particles occur [24]. Then, the coating material having high adsorptive properties and large specific surface area gives the liquid-solid system the desirable flow characteristics (Figure 1).
The wettability of the compacts by the dissolution media is one of the proposed mechanisms for explaining the enhanced dissolution rate from the liquid-solid compacts. Non-volatile solvent present in the liquid-solid system facilitates wetting of drug particles by decreasing interfacial tension between dissolution medium and tablet surface [25].
Components of liqui-solid compact
Formulation: Liqui-solid compact mainly includes
• Non-volatile solvent
• Disintegrants
• Drug candidate
• Carrier material
• Coating material
Non-volatile solvent: Non-volatile solvent should be inert, high boiling point, preferably water-miscible and not highly viscous organic solvent systems and compatible with having ability to solubilise the drug. The nonvolatile solvent acts as a binding agent in the liquid-solid formulation [26]. Various non-volatile solvents used for the formulation of liquid-solid systems include Polyethylene glycol 200 and 400, glycerine, polysorbate 80 and propylene glycol.
Disintegrants: Super-disintegrants increases the rate of drug release, water solubility and wettability of liquidsolid granules. Mostly Super-disintegrants like sodium starch glycolate and crosspovidone are used.
Drug candidates: This technique was successfully applied for low dose BCS class II and class IV drugs which are poorly water soluble and have slow dissolution rate. Examples of drug candidates include Carbamazepine, Famotidine, Piroxicam, Indomethacin, hydrocortisone, naproxen and Prednisolone, Digoxin, Digitoxin, Prednisolone, Hydrocortisone, Spironolactone, Hydrochlorothiazide, Polythiazide, and other liquid medications such as Chlorpheniramine, water insoluble vitamins, fish oil etc.
Carrier materials: Carrier material should be porous material possessing enough absorption properties which contributes in liquid absorption. The carrier and coating materials can retain only certain amounts of liquid and at the same time maintain acceptable flow and compression properties hence, increasing moisture content of carrier’s results in decreased powder flow ability. These include grades of microcrystalline cellulose such as avicel PH 102 and avicel PH200, lactose, eudragit Rl and eudragitRS12 (to sustain drug delivery) etc.
Coating materials: Coating material should be a material possessing fine and highly adsorptive particles which contributes in covering the wet carrier particles and displaying a dry-looking powder by adsorbing any excess liquid. Coating material is required to cover the surface and so maintain the powder flowability. Coating material includes silica (Cab-O-Sil) M5, Aerosil 200, Syloid, 244FP etc.
Formulation of liqui-solid compact: The formulation part of liquid-solid compact mainly includes Pre-formulation studies and Formulation of liquid-solid compact system.
Pre-formulation studies
• Determination solubility of drug in different non-volatile solvents.
• Determination of angle of slide
• Determination of flowable liquid retention potential (Φ value)
• Calculation of Liquid Load Factor (Lf)
• Liqui-Solid Compressibility Test (LSC)
• Solubility studies
Solubility studies are carried out by preparing saturated solutions of drug in non-volatile solvent and analyzing them spectro-photometrically. Saturated solutions are prepared by adding excess of drug to non-volatile solvent and shaking them on shaker for specific time period under constant vibration. After this, the solutions are filtered and analyzed spectro-photometrically.
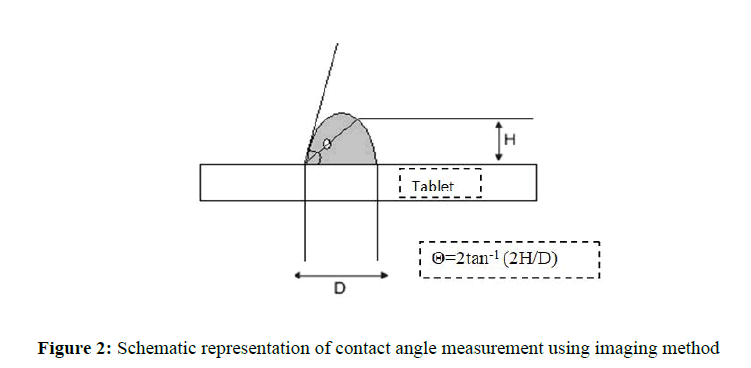
Determination of angle of slide: Angle of slide is used as a measure of the low properties of powders.Determination of angle of slide is done by weighing the required amount of carrier material and placed at one end of a metal plate with a polished surface. The end is gradually raised till the plate becomes angular to the horizontal at which powder is about to slide.This angle is known as angle of slide. Angle of 33° is regarded as optimum(Figure 2).
Determination of flowable liquid retention
Potential (Φ value): The term "flowable liquid-retential potential"(Φ-value) of a powder material describes its ability to retain a specific amount of liquid while maintaining good flow properties. The Φ-value is defined as the maximum weight of liquid that can be retained per unit weight of the powder material in order to produce an acceptably flowing liquid/powder admixture. The Φ values are calculated according to equation
Φ value=weight of liquid/weight of solid … (1)
Calculation of Liquid Load Factor (Lf): Different concentrations of non-volatile solvents are taken and the drug is dissolved. Such liquid medication is added to the carrier coating material admixture and blended. Using equation (2) drug loading factors are determined and used for calculating the amounts of carrier and coating materials in each formulation.
Lf=weight of liquid medication/weight of carrier material… (2)
Liqui-Solid Compressibility Test (LSC): Liqui-solid compressibility test is used to determine Φ values and involves steps such as preparing carrier coating material admixture systems, preparing several uniform liquid or powder admixtures, compressing each liquid or powder admixtures to tablets, assessing average hardness, determination of average liquid content of crushed tablets, as well as determining plasticity, sponge index and Φ value and Lf.
Preparation of liqui-solid tablets
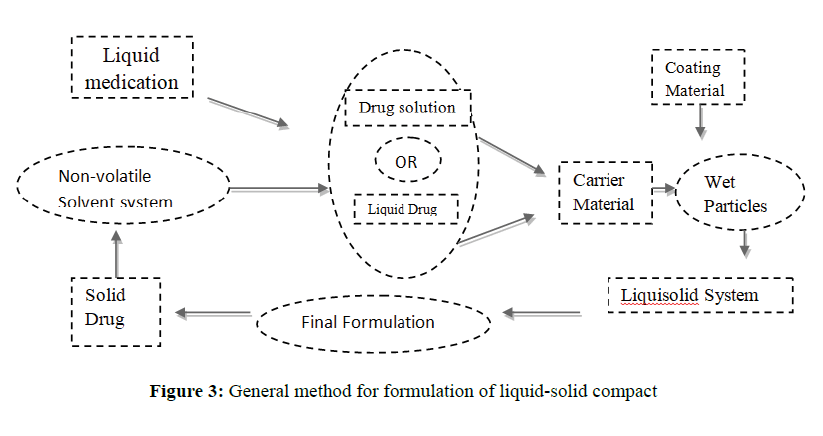
General method of preparation of liquid-solid: A drug substance was initially dispersed in the non-volatile solvent systems (Polysorbate 80, Polyethyleneglycol-200) termed as liquid vehicles with different drug: vehicle ratio.
• Then a mixture of carrier or different polymers and excipients were added to the above liquid medication under continuous mixing in a mortar. These amounts of the carrier and excipients are enough to maintain acceptable flow and compression properties.
• To the above binary mixture disintegrant like sodium starch glycolate and other reaming additives were added according to their application and mixed for a period of 10 to 20 min. in a mortar.
• The final mixture was compressed using the manual tableting machine to achieve tablet hardness.
• Characterize the final liquid-solid granules for solubility, dissolution, flowability, compressibility and other physicochemical properties(Figure 3).
Result and Discussion
Evaluation of liqui-solid systems
Flow behaviour: Flow properties are the important concern in the formulation and industrial production of tablet dosage form. Angle of repose is characteristic to the flow rate of powder. In general, values of angle of repose ≥ 40° indicate powders with poor flowability.
Differential Scanning Calorimetry (DSC): It is necessary to determine any possible interaction between excipients used in the formulation. This will also indicate success of stability studies. If the characteristic peak for the drug is absent in the DSC thermo-gram, there is an indication that the drug is in the form of solution in liquidsolid formulation and hence it is molecularly dispersed within the system
X-Ray Diffraction (XRD): Generally, disappearance of characteristic peaks of drug in the liqui-solid formulation and retaining peaks of carrier material is observed. This indicates that drug gets converted to amorphous form or in solubilised form in the liquid-solid formulation.
Scanning Electron Microscopy (SEM): After SEM study, complete disappearance of crystals of drug which confirms that drug is totally solubilised in liquid-solid system and this ensures the complete solubility. After complete formulation, Tablets are evaluated by carrying out tests for weight variation, uniformity of tablet thickness and diameter, humidity content using Karl-fisher method, friability, hardness, disintegration, dissolution, and content uniformity [27]. All these tests are carried out in triplicate and according to the compendial specifications. For content uniformity test tablets should contain not less than 95% and not more than 105% of the labelled potency. The disintegration test was carried out on six tablets in distilled water at 37°C ± 2°C using the USP disintegration apparatus.
Dissolution studies of liqui-solid tablet: Generally dissolution studies of liqui-solid tablet are carried out using dissolution apparatus USP II at 37°C ± 2°C. Many researchers revealed that at low drug concentrations in liquid medication, more rapid release rates are observed. The consistent and higher dissolution rate displayed by liquidsolid compacts will improve the absorption of drug from gastro-intestinal tract.
In vivo evaluation of liqui-solid tablets: The improvement in oral bioavailability was confirmed by estimating the pharmacokinetic parameters in various animals such as rabbit, beagle dog. Results show that absolute bioavailability of drug from liquid-solid tablets was much higher than marketed tablets.
Conclusion
The liqui-solid technique is a new technique for medications that have an issue with solubility. This method has been shown to improve the dissolving of medications that are poorly water soluble. One of the technical issues in designing a suitable dosage form for optimal medication administration is the solubility of several active pharmacological components. The majority of hydrophobic medications classified as sparingly soluble, slightly soluble, or very slightly soluble disintegrate poorly in the gastro intestinal tract, resulting in unpredictable and partial absorption.
References
- Yadav VB, Yadav AV. J Pharm Sci Res. 2009; 1, 44-51.
- Javadzadeh Y, Siahi MR, Barzegar-Jalali M, et al. J Pharm Sci. 2005; 8, 18-25.
- Spireas S, Jarowski CI, B Rohera. Pharm Res. 1992; 9, 1351-1358.
- Shinde AJ. Pharma Infonet. 2007.
- Manogar PG. J Pharm Sci Res. 2011; 3(12), 1604-1611.
- Patravale VB, Date AA, Kulkarni RM. J Pharm Pharmacol. 2004; 56, 827-840.
- Jaiswal SB and Brahmankar DM. J Biopharm. 1999; 25, 165.
- Kesisoglon F, Panmai S. Adv Drug Dev Rev. 2007; 59, 631-644.
- Spireas S, Sadu S. Int J Pharm. 1998; 166, 177-188.
- Spireas S, Wang T, Grover R. Drug Dev Ind Pharm. 1999; 25, 63-168.
- Gavali MS, Pacharane SS, Sankpal SV, et al. Int J Res Pharm Chem. 2011; 1(3).
- Javadzadehab Y, Navimipour BJ, Nokhodchibc A. Int J Pharm. 2007; 341, 26-34.
- Nokhodchi A, Javadzadeh Y, Siahi MR et al. J Pharm Sci. 2005; 8, 18-25.
- Kulkarni AS, Aloorkar NH, Mane MS, et al. Int J Pharm Sci Nanotech. 2010; 3 (1), 76-98.
- Spireas S, Bolton M. J Chem. 2000; 6 (96), 337.
- Spireas S. US Patent. 2002; 6(423), 339.
- Fahmy RH, Kassem MA. Eur J Pharm Biopharm. 2008; 69, 993-1003.
- Naseem A, Olliff CJ, Martini LG, et al. Int J Pharm. 2004; 269, 443-450.
- Gursoy RN, Benita S. Biomed Pharm. 2004; 58, 173-182.
- Yadav VB. Int J Pharma Res. 2010; 12(1)
- Javadzadeh Y, Musaalrezaei L, Nokhodchi A. Int J Pharm. 2008; 362, 102-108.
- Tayel SA, Soliman II, Louis D. Eur J Pharm Biopharm. 2008; 69, 342-347.
- Yadav VB. J Pharm Res. 2009; 2(4), 670-674.
- Banker GS, Anderson NL. J Pharm. 1987; 293-345.
- Amit k, jain K, Rammulrajsinh R, et al. J Pharm. 2011; 1(1), 24-27.
- Gonjari ID, Karmarkar AB, Hosmani AH. J Nanomat Bio. 2009; 4 (4), 651-661.
- Yousef J, Siahi MR, Ali SA, et al. Acta Pharm. 2007; 57, 99-109.