Original Articles: 2021 Vol: 13 Issue: 8
Formulation and Characterization of Letrozole Based Gold Nanoparticles
Abstract
Gold nanoparticles have been used in biomedical fields since their discovery. Gold nanoparticles are utilized in cancer therapy due to their ability to efficiently deliver therapeutic agents to specific tumor sites. Letrozole is a selective non-steroidal aromatase inhibitor and extremely effective in infertility, breast cancer management and also as adjunct treatment for breast cancer in postmenopausal women. Therefore, Letrozole is loaded in gold nanoparticle to develop a novel system that can efficiently deliver drug to the tumor site without damaging healthy cells. Gold nanoparticles were developed by Turkevich technique and characterized by UV-Vis spectroscopy, DLS (Zetasizer), SEM, TEM, and FTIR. The synthesized particles were identified in the UV range of 520-550 nm. The nanoparticles were spherical in shape having size range of 50-59 nm. This formulation can greatly help for targeted delivery of Letrozole, avoiding harmful side effects on healthy cells and thus provide better treatment in breast cancer.
Keywords
Gold nanoparticles, Letrozole, Breast cancer, Aromatase inhibitor.
Introduction
Conventional chemotherapeutic agents were used to treat cancer in early times, but the main drawback of these conventional drugs was lack of targeting, poor solubility, and their inability to penetrate inside the tumor resulting in decreased treatment with reduced dose and low survival rate (ref). Therefore, novel drug delivery systems were introduced. These systems provide various opportunities to get direct entry into the selective cancer cells, with high concentration of therapeutic agents at the target site, thereby showing minimum side effects [1]. Nanoparticles are especially programmed to recognize the tumor cells and to deliver the selective and precise concentration of therapeutic agents, without any interaction with normal cells. Nanoparticles provide sustained, controlled and targeted delivery of anticancer agents [2,3]. One of the best conducting nanoparticles is metal nanoparticles, e.g., gold NPs, silver NPs, lead NPs etc. These are metal particles with nanometer size and different proportions of length, width and thickness that lie in 1-100 nm size range. Metal NPs are basically sub-micron particles buildup of original gold or silver metals or by their compounds e.g., oxides and chlorides. Silver and AuNPs are well-known due to their optical and thermal properties. Surface plasmon resonance is the fact that gives color to these NPs [4]. These AuNPs are considered as appropriate carriers for the synthesis of novel drug delivery systems. They are biocompatible, easily synthesized and functionalized [5]. These NPs can be synthesized by different chemical methods and techniques like Turkevich method, burst biphasic method, seed-mediated growth and digestive ripening method [6,7].
AuNPs are used in various biomedical fields especially as diagnostic and therapeutic agents due to their high loading efficiency, targeted delivery and good stability [8]. Furthermore, another unique property is their enhanced permeability and retention effect, which allow the AuNPs to accumulate in tumor cells and thus enhance contact time [9]. Therefore, AuNPs are also employed in targeted drug delivery in malignant brain tumors by passive or active targeting. AuNPs have various applications in multiple grounds such as medicine and chemistry due to their exclusive properties like biocompatibility, optical tunability, thermal and simple functionalizable nature [10].
There are various methods and techniques to produce AuNPs, using various sources such as plants, algae, fungi, microorganisms or synthetically by chemical agents. The technique is important to control the size range of produced AuNPs e.g. Turkevich method yields spherical nanoparticles with size range of 5-150 nm [11]. Synthetic methods involve the reduction of metal salts from reducing-agents by standard reaction conditions leading to production of spherically shaped NPs. Common production methods include: Turkevich Method, Brust Biphasic Method, Seeding Method and digestive ripening. Turkevich method was introduced in 1951 [12]. This method was used for the production of spherically shaped NPs within the size range of 10-20 nm. The basic principle of this procedure is the reduction of Au-ions to gold atoms in the existence of reducing-agents such as citric acid, ascorbic acid, formic acid and amino acids or Ultraviolet light. The NPs size is more stabilized by several agents used for capping and stabilizing [13,14].
Breast cancer (BC) is the most frequent type of cancer identified in postmenopausal women after 50 years old, and is an important cause of women death around the globe. About 1.38 million individuals were detected with BC in 2008. It occurs in both males and females, but it is more usual in females [15].
Different approaches and cytotoxic agents are used to treat postmenopausal BC in women. Some agents are used to inhibit certain signaling pathways in abnormal cells which promote their growth. Targeted delivery is also used to enhance drug delivery to the tumor site and to reduce harmful effects on normal cells. Anti HER-II agents act upon the HER-II receptor to inhibit signals and reduce cell propagation in HER-II positive BCs (e.g., trastuzumab, pertuzumab, trastuzumab emtansine, neratinib, and lapatinib) [16]. Similarly, inhibitors of cyclin-dependent kinases (CDK) agents reduce cellular proliferation in tumors (e.g. palbocilib, abemaciclib and ribociclib) [17].
Inhibitors of Mechanistic Target of Rapamycin (mTOR) are used to inhibit the progress and production of tumor cells which are stimulated by mTOR signaling (e.g. everolimus) [18]. Chemotherapy is applied to destroy cancerous cells and to treat triple negative, HER-II positive and luminal-B type of tumors. It is generally continued for about 3 weeks as intravenous infusions. In certain cases, oral chemotherapy is completed with offering standard intravenous chemotherapy.
Endocrine therapy is used to treat BC caused by hormonal factors. It is basically aimed to reduce estrogen and progesterone levels. This is a very important type of systemic therapy for estrogen receptor-positive tumors, known as hormone-dependent tumors [19]. The endocrine therapies can be taken orally or as intravenous administration. Selective estrogen receptor modulators (SERM) inhibit estrogen at the tumor cells that avoid estrogen attachment with the receptor (e.g. Tamoxifen) [20]. Selective estrogen receptor down regulators (SERD) takes part to decrease the number of ER receptors. Gonadotropin-releasing hormone analogues are used to reduce the ovarian function in order to stop the supply of estrogen towards the tumor site [21]. Aromatase inhibitors are used to suppress the estrogen production in muscles and organs except ovaries and therefore they are very efficient in post-menopausal women (e.g. Anastrozole, Exemestane and Letrozole) [22].
In this study, it was decided to develop AuNPs which have good biocompatibility, bioavailability and can be used in passive and active targeting of cancer tissue/cells. Generally, a cancerous tissue displays leaky vasculature around it. This leaky nature of vessels facilitates the entry and accumulation of gold nanoparticles which leave the circulation and fall into such diseased tissues or cells due to their small size. The size of gold nanoparticles is usually smaller than the fenestrations created in the leaky vasculature. In other words, gold nanoparticles are quite a good candidate to utilize the enhanced permeability and retention effect (EPR) occurring in the body. The aim was to load gold nanoparticles with anti-cancer drug Letrozole so that post-menopausal breast cancer can be targeted in a passive manner effectively. The formulated gold nanoparticles were evaluated for their size, size distribution, morphology, drug loading capacity and drug release profile.
Materials and Methods
Materials
Tri-sodium citrate, Hydrogen tetrachloroaurate, Sodium hydroxide were purchased from Sigma Aldrich. Letrozole was received as gift sample from Sun Pharmaceutical Industries. Freshly prepared distilled water and citric acid was obtained from central research lab, The University of Lahore. All the reagents used were of analytical grade.
Method of preparation
Letrozole loaded AuNPs (Letrozole-AuNPs) were synthesized by citrate reduction method described by Joao Conde et al. [23].
Gold nanoparticles were prepared as follows: The gold solution was added into 500 ml of distilled water and heated under continuous stirring. Upon boiling of the solution, sodium citrate solution was added under continuous stirring. The solution color turned into deep red. The solution was kept under continuous stirring for about 30 minutes and was also protected from light. AuNPs were formed and stored in amber color glass bottles.
Preparation of gold solution: 225 ml of 1 mm hydrogen tetrachloroaurate or 0.076 g was dissolved in 225 ml of distilled water.
Preparation of citrate solution: Citrate solution was prepared by dissolving 500 mg of sodium citrate in 500 ml of distilled water under constant stirring.
Letrozole loaded gold nanoparticles are prepared as follow: 20 mg of Letrozole were dissolved in 2 ml of acetone. After their complete dissolution, the solution was transferred into AuNPs solution under continuous stirring for 24 hours. The solution was stored at dark place to avoid aggregation by reaction with light. The loaded AuNPs were collected by centrifugation at 1500 rpm for 45 min in order to separate the blank drug. The loading efficiency was calculated by using the following formula:
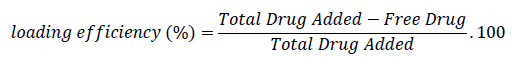
Characterization
The AuNPs were analyzed by using different analytical techniques, such as UV-Visible spectroscopy, DLS Zeta sizer, XRD, SEM, and FTIR. Particle Size and Zeta-Potential analysis.
Mean diameter and polydispersibility index were measured by using photon correlation spectroscopy (PCS) using a Malvern® Zeta sizer. 3000 (Malvern Instruments, GB) at a fixed angle of 90° and temperature of 25°C using distilled water as dispersant. Zetasizer is significant for the determination of particle size and size distribution (polydispersibility, monodispersity) while zeta-potential gives an idea about the particles surface charge which affects their stability.
UV-visible spectroscopy
UV-Vis spectroscopy is usually the first technique used to characterize AuNPs because of the presence of Surface Plasmon Resonance (SPR) phenomenon. SPR is a phenomenon which occurs at the surface of gold, silver or other metallic nanoparticles. In this phenomenon, when an incident light beam strikes the surface at a certain angle, the SPR phenomenon results in a graded reduction in intensity of the reflected light. Thus, make it possible to measure accurately the absorption of molecules on the metal surface [24]. The UV-V is absorption spectra for AuNPs were recorded in the visible range of (510 – 550 nm) which is due to presence of surface plasmon resonance effect [25].
Stability study
The stability of AuNPs was determined by change in particle size. The AuNPs were stored for about 6 weeks and in this period of time, samples were taken and size was analyzed by Dynamic Light Scattering (Zeta sizer).
FTIR analysis
This is an analytical technique which utilizes infrared light to identify organic, polymeric and in certain conditions, inorganic materials. FTIR spectra produce a profile of test sample known as distinctive molecular fingerprint. This molecular fingerprint is used to identify functional groups present in the sample. FTIR spectra of pure active, blank AuNPs and loaded AuNPs were recorded. The FTIR analysis was performed over the range between 1000 and 4000 cm-1.
Colloidal stability
After AuNPs synthesis, the salt and sugar tests were performed. They involve easy steps to investigate the presence of AuNPs. To perform salt tests, three test tubes were taken and named as A, B and C, each containing about 2-3 ml of gold solution. Few drops of NaCl (0.2 g/ml) solution were added in two different test tubes, respectively. While third test tube was kept untreated. The sugar solution was prepared by dissolving 2 g of sucrose sugar in 10 ml of water. Then 3-4 drops of this solution were added to AuNPs in two different test tubes and shaken properly.
Morphology analysis
TEM technique was used to determine the morphology of NPs or to analyze size; shape and distribution of NPs. Different samples were collected and examined at 200 kV under electron microscope. SEM is a surface imaging technique that is used to determine particle size, size distribution and overall surface morphology of synthesized NPs at the micro and nano-scales.
In Vitro drug release study
Right after the preparation of AuNPs, fresh samples were centrifuged for about 30 minutes. The centrifuged sample was added to 50 ml of phosphate buffer solution at a maintained pH of 7.4. The drug release study was carried out at normal physiological temperature of 37°C under continuous stirring at 150 rpm. Samples were taken after a predetermined interval of every one hour and replaced with equal amount of phosphate buffer solution. The amount of released Letrozole was analyzed by using UV-Visible spectroscopy at 337 nm. The same procedure was performed with acetate buffer at pH 4.5.
Results and Discussion
Particles characterization
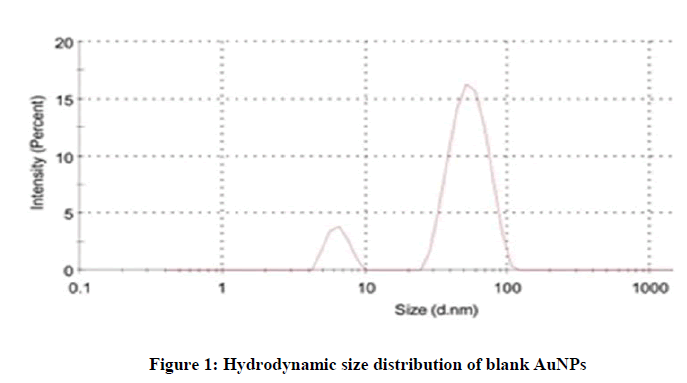
Hydrodynamic particle size and size distribution: The size distribution studies were carried out by dynamic light scattering. The samples were kept at 4℃ before analysis. The average hydrodynamic particle size of blank AuNPs was found to be 54.81 nm which was the average of two populations. The first peak can be attributed to real size, whereas, the second peak can be attributed to aggregated gold nanoparticles (Figure 1).
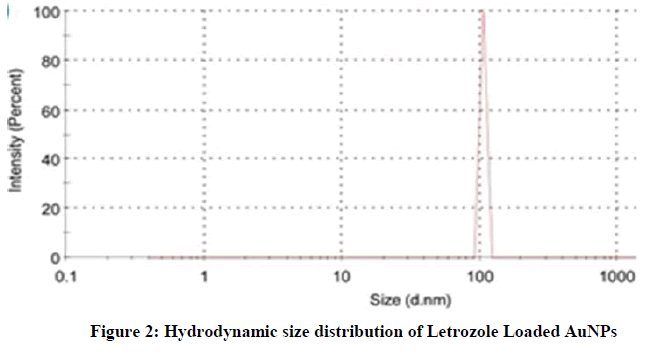
On the other hand, the particle size of Letrozole loaded AuNPs was found to be approximately 105 nm. The observed narrow size distribution is attributed to the specific treatment during Letrozole loading (Figure 2).
Zeta potential
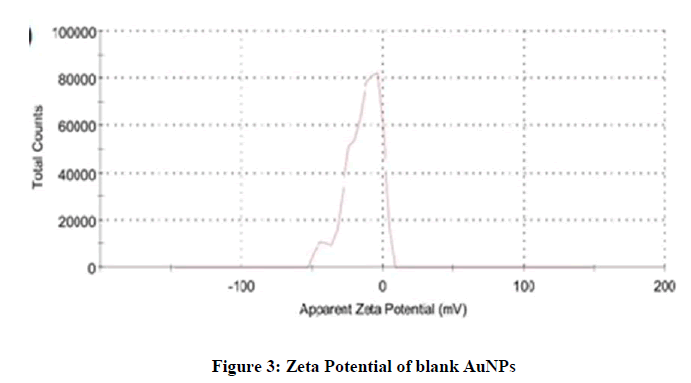
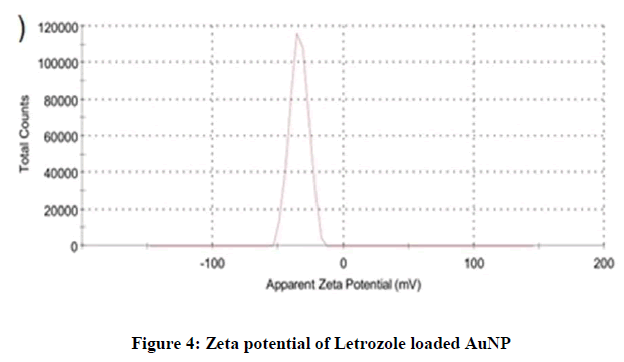
For zeta potential measurement, the samples were run about three times and the average was noted. The measured average zeta potential of blank AuNPs was -14.1 mV, while the zeta potential of Letrozole loaded AuNPs was -33.7. The negative charge was due to the presence of citrate ions at the outer surface of AuNPs (Figures 3 and 4).
The zeta potential of loaded Letrozole-AuNPs was higher than the blank AuNPs due to addition of Letrozole molecules which have three nitrogen (negatively charged) atoms in its chemical structure. Thus, an increase in zeta potential of loaded Letrozole-AuNPs was noted.
UV-Visible spectroscopy
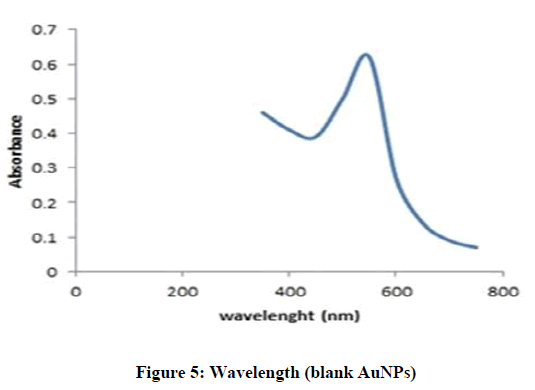
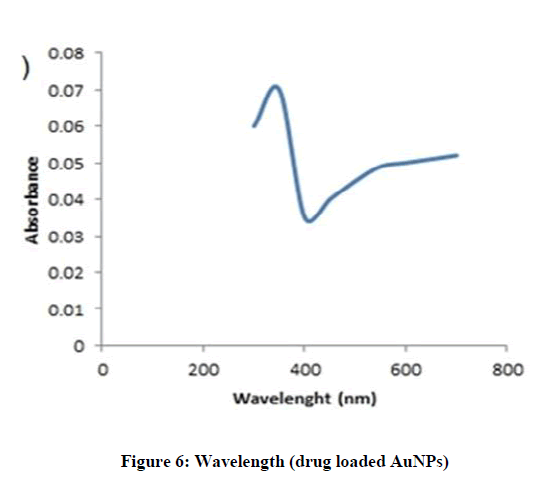
The blank and drug loaded AuNPs were characterized by UV-Vis spectroscopy. In UV-Vis spectra, the distinct peaks and deep red color was observed due to Surface Plasm on Resonance Band, which confirmed the presence of AuNPs. In this study, the blank formulation of AuNPs showed maximum absorption wavelength (λ max) at 530 nm [26], while Letrozole loaded AuNPs showed maximum wavelength at 323 nm. This proved that the active molecule was successfully loaded into the nano-particles (Figures 5 and 6).
Stability studies
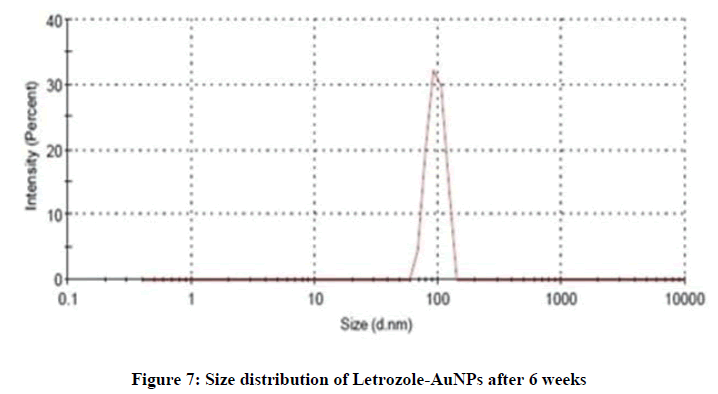
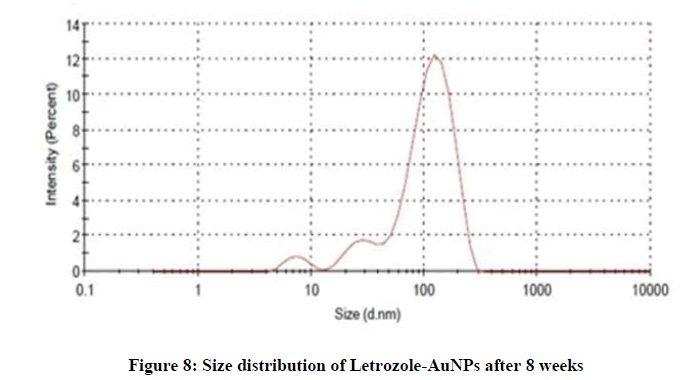
The stability of AuNPs was determined by change in particle size. The AuNPs were stored for about 6 weeks at 4℃ and in this period of time, samples were taken and analyzed by DLS. The average particle size of fresh sample was between 50-60 nm with PDI less than 0.2%, and after 6 weeks it was observed in between 80-90 nm (Figure 7). After 8 weeks the observed size was between 100-110 nm (Figure 8). After the completion of 8 weeks the variation in the size and color of AuNPs indicated good stability of AuNPs. The particle size was a bit increased due the formation of aggregates in the solution that turned the formulation from deep red to slightly purple in color.
Colloidal stability
On addition of NaCl salt, the solution turned into deep blue or purple color. This change was due to the agglomeration of AuNPs, and the particles absorbed longer wavelengths of green, yellow, orange and red, but failed to absorb shorter wavelengths like blue and purple. Therefore, the gold colloidal solution turned into color blue. The color changes are shown in Figures 9A-9C.
Upon addition of sugar solution, no obvious change in color of gold solution was observed. When weak layer/ non-electrolyte like sugar was added to the gold solution, the anionic citrate ion coating was not disrupted and hence the gold solution showed a slight change in color as shown in Figure 10.
FTIR analysis
FTIR spectra of the prepared samples of AuNPs were recorded. Samples were scanned and recorded at transmittance mode at a scanning range of 1000-4000 cm-1.
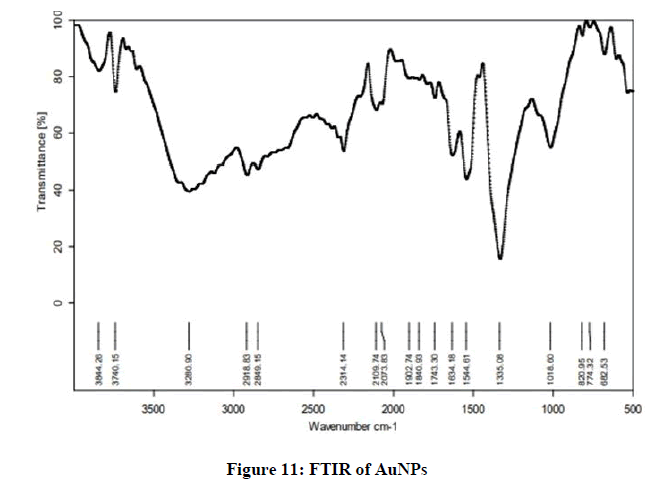
FTIR of AuNPs
The FTIR spectrum of synthesized AuNPs shows availability of functional groups. The recorded spectrum shows broad absorption peaks at 3844.26 cm-1, 3740.15 cm-1, 2109.74 cm-1, 1544.61 cm-1, 1335.08 cm-1 and 774.32 which indicate the presence of O-H stretch hydrogen bond, -C≡C- stretch alkyne bond, C=C stretch alkene, and C-H bending bond, respectively. These peaks are visible in Figure 11.
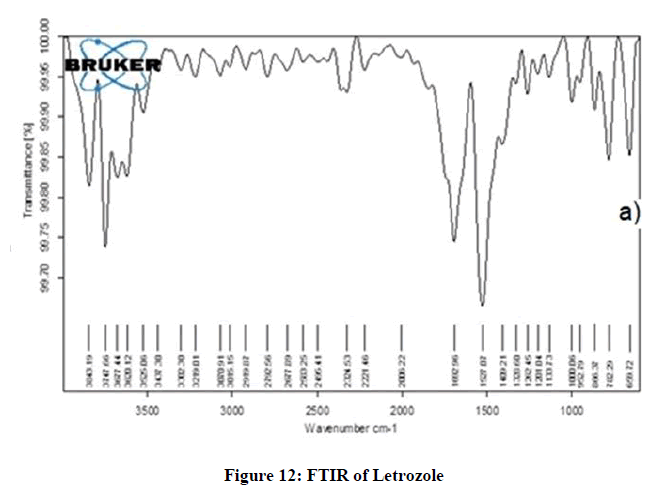
FTIR analysis of Letrozole
The FTIR spectrum of pure Letrozole was recorded. The spectrum revealed different peaks at 3725.25 cm-1, 3677.44 cm-1, 3525.06 cm-1, 3302.20–2300 cm-1, tiny absorption peaks at 1328.88-1135.88 cm-1 and 782-685.45 cm-1 which indicated the presence of O-H strong hydrogen stretch bond, N-H stretch bond, cyclic ring, C=C stretch alkene, C-H bending and C-N stretch nitrile, respectively [27] (Figure 12).
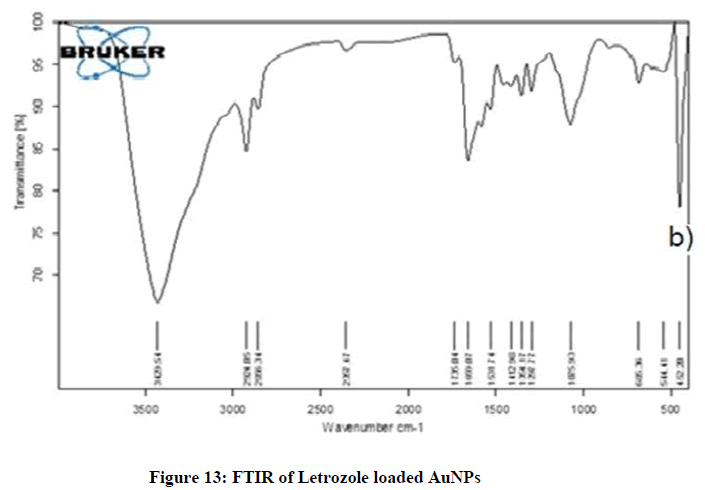
FTIR of Letrozole loaded AuNPs
The FTIR spectrum of Letrozole loaded AuNPs showed characteristic peaks at 3863.19 cm-1, 2994.05 cm-1, 2319.47 cm-1, tiny absorption peaks at 1706.04-1219.77 cm-1, then 1085.90 cm-1, characteristic peaks were observed between 690.36-544.41 cm-1 which indicated the presence of O-H stretch hydrogen bond, -C ≡ C stretch variable bond, C=C stretch variable and C-H strong stretch bond, respectively. The FTIR spectrum of Letrozole loaded AuNPs displayed characteristic peak of active at 3863.19 cm-1 (N-H), 2994.05 cm-1 (N-H), 2319.47 cm-1 (C ≡ N) stretched, 2319.47 cm-1 (C ≡ C) stretched, and 1219.77 cm-1 (C-O) bending while the AuNPs showed (3320.54 cm-1 (O-H) stretched, 1637.22 cm-1 (C=C) stretched and 689.89 cm-1 (C-X) bending] which confirmed the loading of cyclophosphamide in AuNPs. The spectrum of AuNPs was similar to the spectrum of Letrozole loaded AuNPs which represented the proper loading of drug in AuNPs. Characteristic peak of Letrozole (as seen in spectrum of drug) showed low intensity and were concealed or hidden under the spectrum of FTIR in Figure 13.
Morphology analysis
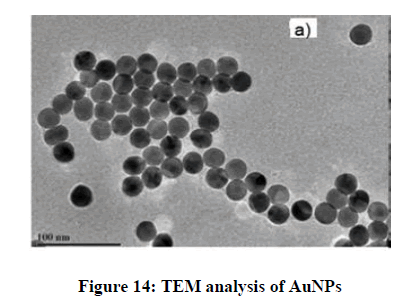
The size and morphological structure of the prepared AuNPs were investigated by TEM. The results revealed the presence of highly spherical and monodispersed particles having ultra-smooth surface, (can you estimate the size and size distribution from this TEM image) shown in Figure 14.
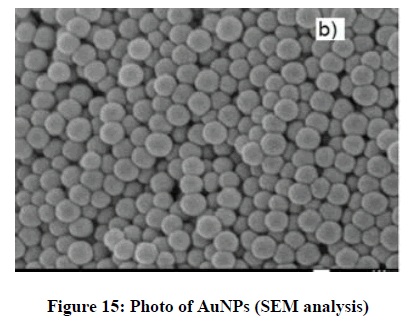
From SEM analysis, it was observed that AuNPs were spherically shaped. The size range for spherical AuNPs was found to be between 54 and 100 nm. Moreover, the SEM images showed monodispersity of the AuNPs, as shown in Figure 15.
In Vitro drug release study
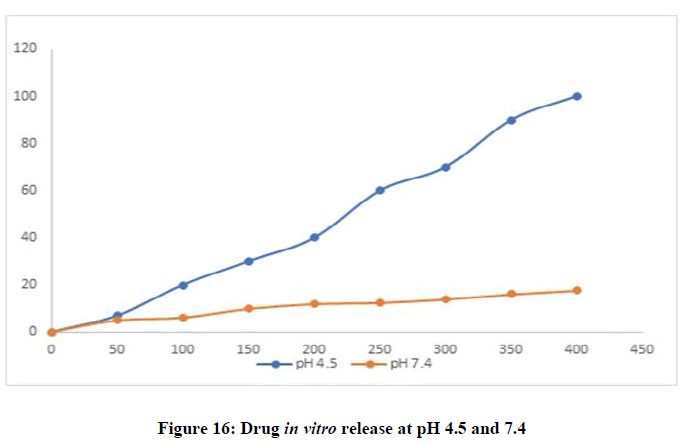
A pH-responsive drug release was observed which is beneficial in tumor treatment. The Letrozole-AuNPs showed very little release at physiological pH 7.4 while it showed a burst drug release at acidic pH 4.5. As the pH of tumors range from 4 to 6.8 which is much lower than normal physiological pH, Letrozole-AuNPs would provide better release at tumor site without harming healthy cells. While, the loading efficiency of Letrozole in AuNPs was found to be 73 ± 5% which meant that 730 μg of Letrozole was loaded in 1 mL of AuNPs (Figure 16).
Conclusion
The gold nanoparticles loaded with Letrozole were prepared by Turkevich method (citrate reduction). The resulted Letrozole loaded AuNPs displayed spherical morphology and particle size of approximately between 50-59 nm. These particles were characterized by UV-visible spectroscopy, zeta sizer, SEM, TEM, and FTIR. The in vitro release pattern showed good solubility at acidic pH which is similar to tumor pH. This ensures their targeted release at tumor site. Further studies are required on in vivo drug release mechanism. Meanwhile stability studies were performed for the period of 6 weeks, which revealed their good stability at 4ºC.
However, these Letrozole loaded AuNPs will have great potential applications in medicine, especially in the treatment post-menopausal breast cancer. Hence, this formulation can greatly help in targeted delivery of Letrozole, while avoiding harmful side effects on healthy cells.
Future prospects
The developed Letrozole loaded AuNPs will have great potential applications in the field of medicine, further studies are required on its stabilization, mechanism of action, ligands and biomarkers can also be used to ensure site specific delivery. Cell line studies may provide more intense information about its pharmacological outcomes.
References
- Utreja P, Jain S, Tiwary A K. J Anti-cancer Drugs. 2010; 7(2), 152-61
- Basen-Engquist K, Chang M. J Oncology. 2011; 13(1), 71-6.
- Sutradhar KB, Amin ML. ISRN Nanotechnology. 2014
- Fedlheim DL, Foss CA. Metal nanoparticles. 2001.
- Austin LA, Mackey MA, Dreaden EC, et al. Bio-Diagn Drug Delivery. 2014; 88(7), 1391-417
- Prasad B, Stoeva SI, Sorensen CM, et al. Nanoparticles Chem Thiols. 2003; 15(4), 935-42.
- Hanžić N, Jurkin T, Maksimović A, et al. Radiation Phy Chem. 2015; 106, 77-82.
- Menon S, Rajeshkumar S, Kumar V. Resource-Efficient Techn. 2017; 3(4), 516-27.
- Nakamura Y, Mochida A, Choyke PL, et al. Nano Drug Delivery. 2016; 27(10), 222-538.
- Sperling RA, Gil PR, Zhang F, et al. J Nano Chemical Soci. 2008; 37(9), 1896-908.
- Gorup LF, Longo E, Leite ER, et al. J Colloid Interface Sci. 2011; 360(2), 355-8
- Turkevich J, Stevenson PC, Hillier. J. Discussions Faraday Soci. 1951;11, 55-75.
- Maruyama T, Fujimoto Y, Maekawa T. J Colloid Interface Sci. 2015; 447, 254-257.
- Kimling J, Maier M, Okenve B, et al. J Phy Chem Bio. 2006; 110(32), 15700-7.
- Winters S, Martin C, Murphy D, Shokar NK. J Mol Biology Trans Sci. 2017; 151, 1-32.
- Minckwitz GV, Procter M, De Azambuja E, et al. New England J Med. 2017; 377(2), 122-31.
- Wee I, Wong ALA, Goh RM, et al. Cochrane Data Sys Rev. 2018; 1
- Lamming DW. J Med. 2016; 6(5), a025924.
- Rugo HS, Rumble RB, Macrae E, et al. J Clinical Oncology. 2016; 34(25), 3069-103
- Miller TE, Ghoshal K, Ramaswamy B, et al. J Bio Chem. 2008; 283(44), 29897-903.
- Howell SJ, Johnston SR, Howell A. J Clinical Endocrinology Metabolism. 2004; 18(1), 47-66.
- Howell SJ, Johnston SR, Howell A. J Clinical Endocrinology Metabolism. 2004; 18(1), 47-66.
- Chumsri S, Howes T, Bao T,et al. J Steroid Biochem Mol bio. 2011; 125(1-2), 13-22.
- Conde J, Oliva N, Artzi N. J Bio Chem. 2015; 112(11), 1278-1287.
- Englebienne P, Van Hoonacker A, Verhas M. J Spectroscopy. 2003; 17(2, 3), 255-73
- Suman T, Rajasree SR, Ramkumar R. J Mol Biomol Spectroscopy. 2014; 118, 11-6
- Song JY, Jang H-K, Kim BS. J Bio Chem. 2009; 44(10),1133-8.