Original Articles: 2024 Vol: 16 Issue: 5
Exploration of Unconventional Holistic Approach for Identification of Potential Leachable using Product Label Contact Study with Ophthalmic Solution
Sandeep Zokande*, Kavita Inamdar, Amit Gosar, Vikas Mane
Indoco Remedies limited, R & D centre, Navi Mumbai, India
Received: 06-Jun-2024, Manuscript No. JOCPR-24-138448; Editor assigned: 10-Jun-2024, PreQC No. JOCPR-24- 138448 (PQ); Reviewed: 24-Jun-2024, QC No. JOCPR-24-138448; Revised: 01-Jul-2024, Manuscript No. JOCPR-24-138448 (R); Published: 08-Jul-2024, DOI:10.37532/0975-7384.2023.16(6).162
Citation: Zokande S, et al. 2024. Exploration of Unconventional Holistic Approach for Identification of Potential Leachable using Product Label Contact Study with Ophthalmic Solution. Res. 16:162.
Abstract
Knowledge of synthetic route of drug substance and degradation pathway of drug product is acceptable approach for listing of impurities under drug product specification. Forced degradation, stress study, drug-excipient compatibility, extractable and leachable profile, literature search, impurities listed in compendial monographs is the end-to-end confirmation of generic drug product specification. The number of recalls of liquid pharmaceutical products from market is rising in comparison to that of solid pharmaceutical products. Failure to meet acceptance criteria of impurity is major concern for these market recalls. Hence, designing of appropriate specification and acceptance criteria of impurities for liquid pharmaceutical products is utmost important. Designing the impurity specification of liquid pharmaceutical products sometimes challenging task due to slow migration of components of container closure system and their low concentration. Even though the information is available about the composition of primary and market packaging components; it is challenge to analytical chemist to decide potential impurities/leachables arising from packaging materials. In this study, two unknown impurities are identified in generic version of liquid drug product applying simple and quick contact study model of drug product with product label. The identification of impurities was further confirmed by isolation, comparison with authenticated standard and by correlating chromatographic and spectral data. This integrated approach can be universally applied for appropriate analytical experimental design which in turn help analytical chemist to identify potential leachables in any liquid pharmaceutical products during product development phase.
Keywords
Container closure system; Packaging component; Leachable; Identification; Impurity
Introduction
Liquid ophthalmic drug products are packed in plastic Container Closure System (herein after it termed as CCS) due to several advantages. Guidance is available to pharma industry on quality control aspects of CCS used for packaging of drug products. However, less attention is given towards control of certain components of secondary CCS such as additives, plasticiser, photo initiators and activator which are used in blend of product label. Due to long-term contact of drug product with immediate CCS, there is possibility of migration of components of CCS in drug product [1-8]. Certain pharmaceutical products may react with such components and accelerates the rate of degradation of drug product and generates toxic impurities hence, selection of appropriate product label is critical. Interaction of drug product with packaging component expected to be investigated and appropriate action is necessary to mitigate probable patient risk. Various regulatory authorities recommend extractable study to identify the potential leachables originating from immediate CCS as extractables using wide range of polarity of solvents [9-15]. Based on extractable study, the leachables above analytical evaluation threshold is included under drug product specification. These leachables are monitored till end of the shelf life of drug product. Researchers also provided procedure to conduct risk assessment of potential leachables originated from immediate CCS [16-19]. Analytical chemists working in pharma industries are experiencing that, recommendations by regulators to identify leachables in liquid pharmaceutical products originating from CCS by extractable study is not sufficient. In this context, commonly used “Diclofenac ophthalmic solution” (hereinafter it termed as generic drug product) having qualitative and quantitative composition similar to innovator (Voltaren) is manufactured. An analytical procedure using high performance liquid chromatography is developed to estimate the impurities. The generic drug product was stored in stability chamber to evaluate the product quality by monitoring critical quality attributes. Near expiry innovator was also analysed to identify and discriminate the product related impurities. During stability study of generic drug product, two unknown impurities found above identification threshold. Crossing of identification threshold of impurity in any drug product invites assigning of reduced shelf life. Hence, to overcome this criticality and to obtain information about impurities arising from product label quick, easy and reliable contact case study experimental model which discriminate the impurities related to drug product and those originates from product label is designed which helps to evaluate the integrity of liquid pharmaceutical product well in advance. The study is focused to identify two unknown impurities originated from product label of generic drug product by applying this holistic approach. These impurities further isolated from generic drug product using preparative High Performance Liquid Chromatography (HPLC).
MATERIALS AND METHODS
Chemicals, standards and samples
HPLC grade methanol, phosphoric acid, monobasic sodium phosphate and sodium phosphate (purity>99%) were purchased from Merck. The ultra-pure water was collected from MilliQ water system (Millipore, France). Nylon filter membranes (diameter=47 mm, pore size=0.45 μm) were obtained from Merck (Germany). The standards (purity>99%) were purchased from authorised manufacturers. Methyl-2-benzoyl benzoate (lot no. QUHXE-DR) was procured from TCI. 1-Hydroxycyclohexyl phenyl ketone (lot no. H62TM-BF) was procured from TCI. Generic drug product “Diclofenac ophthalmic solution” having qualitative and quantitative composition similar to that of innovator “Voltaren” was manufactured. The generic product was manufactured containing diclofenac sodium 0.1% (1 mg/mL) as an active and polyoxyl 35 castor oil, boric acid, tromethamine, sorbic acid (2 mg/mL), edetate disodium (1 mg/mL) as an inactive and purified water as vehicle. The generic drug product was filled in Light Density Polyethylene (LDPE) bottles. These bottles were fitted with nozzle and cap. The printed product labels were affixed on each bottle. The placebo was manufactured using similar process followed to manufacture generic drug product excluding diclofenac sodium. The placebo containing polyoxyl 35 castor oil, boric acid, tromethamine, sorbic acid (2 mg/mL), edetate disodium (1 mg/mL) as an inactive and purified water as vehicle was used to manufacture placebo. The placebo was filled in LDPE bottles. These filled LDPE bottles were fitted with nozzle and cap. The printed product labels were intentionally not affixed to bottles filled in placebo. Diclofenac sodium and excipients used to manufacture ophthalmic solution and placebo were of USP-NF grade and procured from authorised manufacturers. Ink components and varnish used to print product labels were procured from commercial label manufacturer.
Development of HPLC method
The HPLC method was developed using Water’s HPLC and C8 reverse phase column (particle diameter 5 μm, 4.6 mm inner diameter, length 250 mm) at 25ºC with a UV-VIS detector with Diode Array (DAD), at a wavelength of 254 nm. The development of the analytical method considered the evaluation of composition of mobile phase (solvent ratio, polarity), with the objective of baseline resolution of peaks due to diluent, placebo, diclofenac and related impurities. The mobile phase was composed of prefiltered and degassed mixture of 0.01 M sodium phosphate buffer and methanol in ration of (30:70) v/v, permeating with a volumetric flow of 1.0 mL per minute in isocratic mode at 25ºC. The injection size was set as 10 μL.
Preparation of solutions for contact study using product label, generic drug product and placebo
A label from a bottle of generic drug product was carefully removed. The label was cut into small pieces and added into a separate bottle containing placebo. The nozzle and cap were fitted to this bottle. Another bottle of generic drug product was selected and the label was carefully removed. The label was cut into small pieces and added in same bottle containing generic drug product. These mixtures were stored at 25ºC for 12 hours. Simultaneously similar mixtures were prepared using two product labels, placebo and generic drug product and heated these two independent mixtures at 80ºC for 1 hour. The resultant solutions were injected into HPLC system using method described in above section.
Analysis of components of product label
Magenta, yellow, cyan, blue colours and varnish used to print commercial product label of most of the ophthalmic solutions. Identification solutions of these individual colours and varnish (having 1 mg/mL concentration) were prepared using mobile phase as a diluent and injected in to HPLC system using method described in above section.
Development of preparative HPLC method
The preparative HPLC method was developed using Water’s HPLC model 2767, 2998, CFO, 2525. Preparative HPLC column (particle diameter 5 μm, 30 mm inner diameter, length 100 mm) at 25ºC with a UV-VIS detector at a wavelength of 254 nm. 2% glacial acetic acid is used as mobile phase-A and 100% methanol is used as mobile phase-B with the objective of baseline resolution between peak of interest and nearby peaks. The injection size was set as 3500 μL. Optimised gradient program with flow gradient used for isolation of impurities are summarised in Table 1.
| Gradient Program-1 for impurity at Relative Retention Time (RRT) 0.58 | Time (minutes) | Flow Rate (mL/minute) | Mobile phase-A (%) | Mobile phase-B (%) |
| 0 | 25 | 44 | 56 | |
| 13 | 25 | 34 | 66 | |
| 17.5 | 25 | 25 | 75 | |
| 18 | 50 | 5 | 95 | |
| 19 | 25 | 44 | 56 | |
| 21 | 25 | 44 | 56 | |
| Gradient Program-2 for impurity at Relative Retention Time (RRT) 0.66 | 0 | 25 | 45 | 55 |
| 10 | 25 | 35 | 65 | |
| 12 | 30 | 0 | 100 | |
| 13 | 30 | 0 | 100 | |
| 14 | 25 | 45 | 55 | |
| 17 | 25 | 45 | 55 |
Table 1: Gradient program for impurity at Relative Retention Time (RRT).
Development of GC-MS method
The gas chromatograph equipped with mass spectrometer consisting of a Perkin Elmer GC model Clarus 500 with a DB-624 column (Length: 30 m, Id: 0.32 mm, thickness: 1.8 μ) was used. The carrier gas was helium with flow rate of 1.2 mL/minute. The injection size at 0.5 μL with split ratio 10:1 was set. Temperature of injector was set at 200ºC. The oven temperature was initially maintained at 40ºC for 4 minutes and increased to 200ºC for 18 minutes at a rate of 20ºC/minute. The temperature of ion source of electrons was kept at 230℃ and the Quadrupole was set at 150ºC. Mass spectra were measured using EI+ as ion mode.
Results and Disscussion
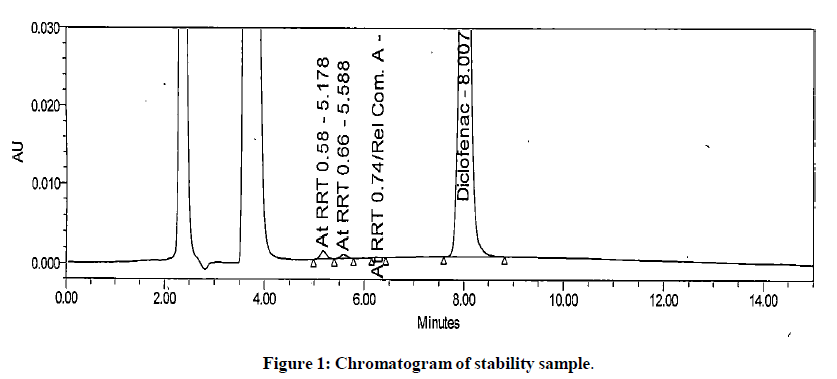
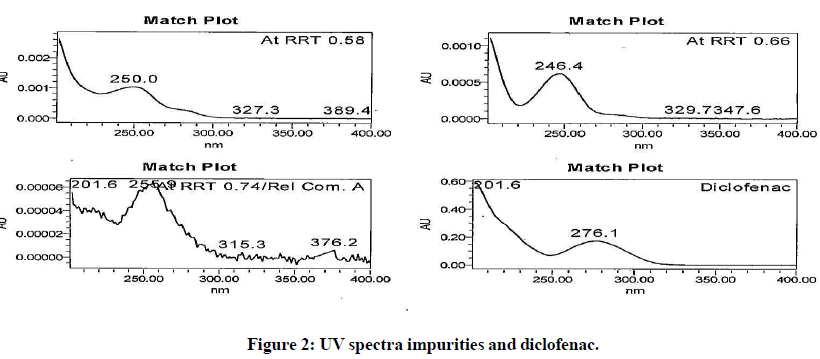
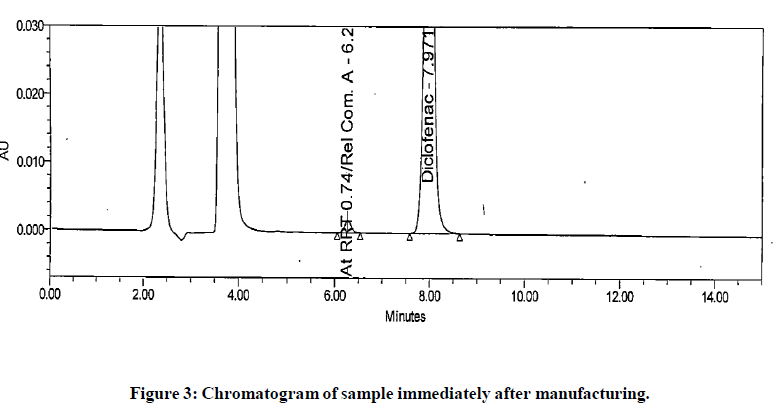
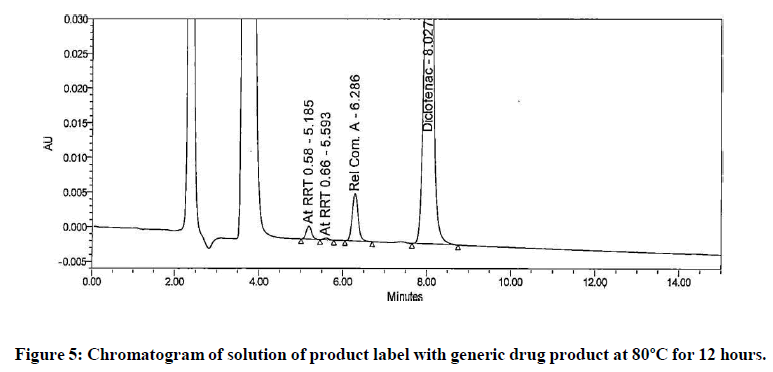
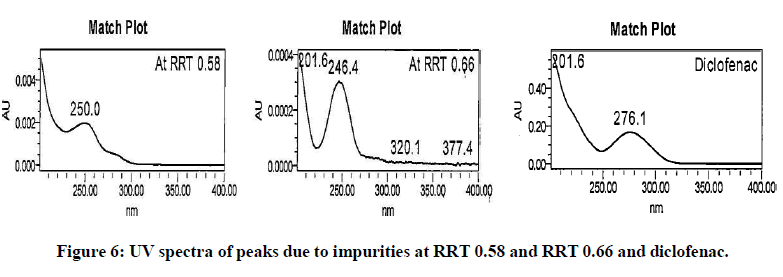
The generic version of “Diclofenac ophthalmic solution” was manufactured and stored in stability chamber to evaluate shelf life and storage instruction. During stability sample analysis two unknown impurities at RRT 0.58 and RRT 0.66 found more than acceptance criteria (>0.2%). The UV (obtained using photo diode array) spectra of these impurities found different than diclofenac and identified impurity. The absence of these impurities in generic drug product immediately after its manufacturing and difference in spectral pattern preliminary indicated that these impurities are not product related. The chromatogram of stability sample is shown in (Figure 1). The UV spectra of these impurities, diclofenac and identified impurity (diclofenac related compound-A) are shown in (Figure 2). The chromatogram of generic drug product obtained during analysis after its immediate manufacturing is also shown in (Figure 3).
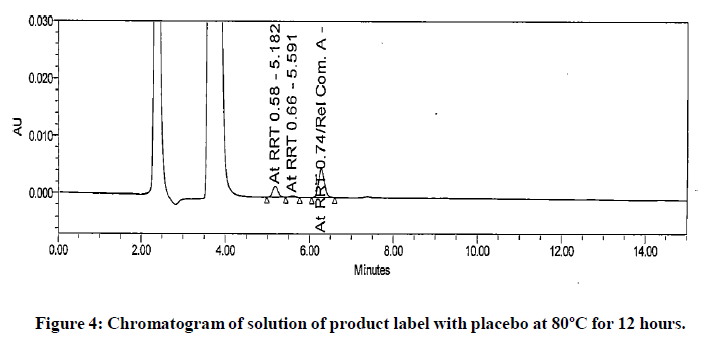
The impurities at RRT 0.58 and RRT 0.66 were not observed during forced degradation, drug excipient compatibility study, stress study and prototype stability study. Hence, it suspected that these impurities might be due to migration of components of product label affixed on product bottle. To investigate this hypothesis generic drug product was manufactured. Contact study between product label, placebo and generic drug product was performed. The preparation of solution for this study is detailed in section in section “preparation of solutions for contact study using product label, generic drug product and placebo”. These solutions were analysed by chromatographic method reported in section “development of HPLC method”. The impurities at RRT 0.58 and RRT 0.66 observed in chromatogram of mixture of product label and placebo. These impurities also observed in chromatogram of mixture of product label and generic drug product. The peak response of these impurities increased in solutions when contact time extended to 12 hours. The peak response of these impurities further increased in the solutions heated at 80℃ for 1 hour. The impurities in mixture of product label and placebo and increased content in both the mixtures when extended contact time at elevated temperature indicated that these impurities are due to migrated components of product label. The chromatograms of heated solutions of product label with placebo and product label with generic drug product at elevated temperature are shown in (Figures 4 and 5). The UV spectra of these impurities are also shown in (Figure 6).
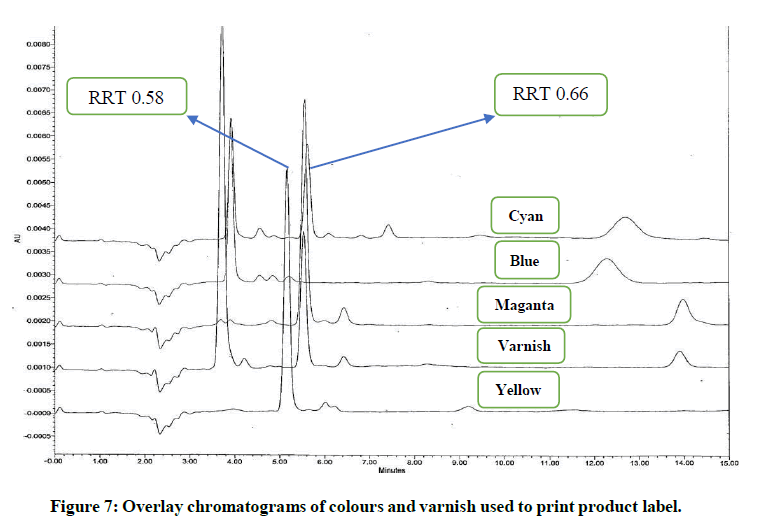
Magenta, yellow, cyan, blue colours and varnish are the major components used to print these product labels. To investigate the exact component responsible for these impurities; identification solutions of these colours and varnish were prepared as per preparation procedure reported in “analysis of components of product label” and analysed using analytical method reported in section “development of HPLC method”. It noticed that the impurity at RRT 0.58 found due to yellow and blue colour. The impurity at RRT 0.66 found due to varnish, magenta and cyan colour. UV spectra of these impurities found similar to those obtained in generic drug product obtained during stability study. The analysis of identification solutions of colours and varnish confirmed the source of these impurities. The overlay chromatograms of colours and varnish (components of product label) are shown in (Figure 7). UV spectra of the peaks elutes at RRT 0.58 and 0.66 from these identification solutions are also shown in (Figure 8).
From contact study it confirmed that unknown components of product label migrated in generic drug product. Hence, to isolate these impurities, preparative HPLC method was developed as reported in section “development of preparative HPLC method”. Two gradient programs were developed. Gradient program-1 was developed for isolation of impurity at RRT 0.58 and gradient program-2 was developed for isolation of impurity at RRT 0.66. Using gradient program-1, generic drug product stored for stability study was injected in multiple replicates. Fractions of impurity at RRT 0.58 were collected and injected in to HPLC using method as reported in section “development of HPLC method” to confirm the correctness and stability of isolated impurity. The isolate was further lyophilised to get impurity in pure form. The isolated impurity was analysed using GC-MS method as reported in section “development of GC-MS method”. The mass spectra obtained from isolated impurity was compared with NIST library. The mass spectra of isolated impurity closely resembled to methyl-2-benzoyl benzoate. To confirm this finding, commercially available standard methyl-2-benzoyl benzoate was used for comparative analysis using UV, HPLC, FTIR and Mass spectroscopy.
The chromatographic and spectroscopic data firmly correlated to that of standard methyl-2-benzoyl benzoate. From this data the impurity at RRT 0.58 was identified as methyl-2-benzoyl benzoate.
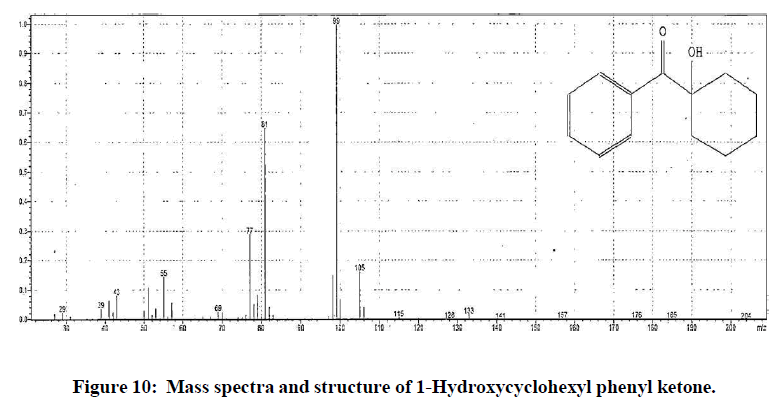
Similarly, gradient program-2 of preparative HPLC was used for isolation of impurity at RRT 0.66. Generic drug product stored for stability study was injected in multiple replicates. Fractions of impurity at RRT 0.66 were collected and injected in to HPLC using method reported in section “development of HPLC method” to confirm the correctness and stability of isolated impurity. The isolate was further lyophilised to get impurity in pure form. The isolated impurity was analysed using GC-MS method as reported in section “development of GC-MS method”. The mass spectra obtained from isolated impurity was compared with NIST library. The mass spectra of isolated impurity closely resembled to 1- Hydroxycyclohexyl phenyl ketone. To confirm this finding, commercially available standard 1-Hydroxycyclohexyl phenyl ketone was used for comparative analysis using UV, HPLC, FTIR and mass. The chromatographic and spectroscopic data corelated to that of obtained using standard 1-Hydroxycyclohexyl phenyl ketone. From these results the impurity at RRT 0.66 was identified as 1-Hydroxycyclohexyl phenyl ketone.
The structures elucidated for methyl-2-benzoyl benzoate (Molecular weight 240 g/mol) and 1-Hydroxycyclohexyl phenyl ketone (Molecular weight 204 g/mol) and their mass spectra are shown in (Figures 9 and 10). Finally, to safeguard patient, the manufacturer of product label was changed by effective implementation of product label contact study model and specific analytical method was developed taking into consideration of such leachables, validated and implemented as a critical quality attribute to evaluate shelf life and storage statement of generic drug product.
Conclusion
A simple and reliable contact study approach has been successfully applied for identification of potential leachables originating from drug product label in short time. UV, FT-IR and GC-MS spectral techniques confirmed the structure of isolated leachable. The comparison with authenticated standard and NIST standard library further confirmed these structures. The approach can be applied as an effective tool in analytical laboratory during development of suitable quality control attribute of liquid dosage forms. The use of placebo further discriminates product related impurities and those originate from packaging components which in turn helps for impurity classification and proposing appropriate acceptance criteria. Use of organic solvents as recommended in extractable study may provide complex information, however this conventional approach by simulating with drug product provides additional benefits to obtain precise information about potential leachables which are soluble in aqueous vehicle used to manufacture most of the liquid pharmaceutical products. The information about most of the constituents used to manufacture product labels are not declared by label manufacturer hence, this tool can be used as reverse engineering to get valuable information about composition of product label. This research has shown that the packaging components plays a vital role to include impurities in specification of liquid drug products. This holistic unconventional approach explored in this study further strengthen the speed of identification process of leachables and has proved great advantage to ascertain patient safety during product development phase itself and helps to avoid potential product recalls.
References
- Guidance for industry. Container closure systems for packaging human drugs and biologics, chemistry, manufacturing, and control documentation. 1999.
- Chunwang Fu, Jiang F, Kun Gao K, et al. J Pharm Biomed Anal. 2023;(235):115591.
[Crossref] [Google Scholar] [PubMed]
- Pan C, Harmon F, Toscano K, et al. J Pharm Biomed Anal. 2008;46(3):520-527.
[Crossref] [Google Scholar] [PubMed]
- Dorival-Gracia N, Larsson I, Bones J. J Pharm Biomed Anal. 2017;142:337-342.
[Crossref] [Google Scholar] [PubMed]
- Liu J, Kennedy JH, Ronk M, et al. Rapid Commun Mass Spectrom. 2014;28(21):2285-2291.
[Crossref] [Google Scholar] [PubMed]
- Liu J, Ronk M, Fujimori K, et al. AAPS Pharm Sci Tech. 2021;22(2):75.
[Crossref] [Google Scholar] [PubMed]
- Jenke D. TrAC Trends Anal Chem. 2018;101:56-65.
- Gollapalli R, Sing G, Blinder A, et al. J Pharm Sci. 2019;108:3187-3197.
[Crossref] [Google Scholar] [PubMed]
- Zokande S, Inamdar K, Kale A. World J Pharm Pharm Sci. 2023;12(4):1600-1607.
- Holm R, Elder DP. Eur J Pharm Sci. 2016;87:118-135.
[Crossref] [Google Scholar] [PubMed]
- Hammond M, Nunn H, Rogers G, et al. PDA J Pharm Sci Technol. 2013;(67):123-134.
[Crossref] [Google Scholar] [PubMed]
- Liu D, Samuel YN, Pavel V, et al. PDA J Pharm Sci and Technol. 2012;66:12-19.
[Google Scholar] [PubMed]
- Singh G, Gollapalli R, Blinder A, et al M. J Pharm Biomed Anal. 2018;152:197-203.
[Crossref] [Google Scholar] [PubMed]
- Vardakou S. Karampela I, Papoutsis C, et al. J Anal Chem. 2014;(69):1096-1101.
- Gracia ND, Bones J. J Chromatogr A. 2017;(1513):69-77.
[Crossref] [Google Scholar] [PubMed]
- European Medicines Agency, International conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use, ICH Q1A (R2), Stability testing of new drug substances and products. Q1-R2. 2003.
- Houston CT, Rodrigues AD, Smith BB, et al. PDA J Pharm Sci Technol. 2022;76:278-294.
[Crossref] [Google Scholar] [PubMed]
- Parris P, Martin EA, Stanard B, et al. Regul Toxicol Pharmacol. 2020;118:104802.
[Crossref] [Google Scholar] [PubMed]
- Johns SM, Jickells SM, Read WA, et al. Packag Technol Sci. 2000;13:99-104.