Original Articles: 2021 Vol: 13 Issue: 3
Economic Microwave Irradiation Technique: Assist the Synthesis of Some Novel 2, 5 -Disubstituted-1, 3, 4-Oxadiazoles and Their Biological Activity
Salim J. Mohammed1*, Shakir M. Saied2 and Bassam T. Khalil2
1 Department of Chemistry, College of Science,University of Mosul, Mosul, Iraq
2 Dentistry Department, Al-Noor University College, Mosul, Iraq
Abstract
The synthesis of 2-substituted-5-(α,α-diphenyl-α-hydroxymethyl)-1,3,4-oxadiazoles(5a-e)by heating benzilic acid hydrazide(3)either with several carboxylic acids in presence of phosphorus oxychloride, or refluxing this hydrazide with corresponding aldehydes to give substituted benzilyl hydrazones(4a-f) However, then oxidative cyclization of those hydrazones with ferric chloride to give the same products(5a-f). Assistance of microwave irradiation for synthesis of benzilyl hydrazones (4a–f) is introduced now a day. In the present study microwave promoted condensation reaction of aromatic aldehydes and benzilic acid hydrazide (3) are displayed, then cyclization by microwave irradiation. The structures of the prepared compounds were characterized through spectral and physical methods. Some of the synthesized products showed good biological activity.
Keywords
Heterocyclic; Hydrazones; Oxadiazole; Biological activity; Microwave irradiation
Introduction
Oxadiazole derivatives are robust pharmaceutical compounds with antiangiogenic and ant proliferative potential both in vitro and in vivo [1], as inhibitors of Mycobacterium tuberculosis[2] in addition to they are good against E. coli, P. aeruginosa, S. aureus and S. pyogenes as compared to standard Ampicillin [3]A modest and well-organized oxidative cyclization of aroyl hydrazones with aldehyde or ketone derivatives enables the synthesis of 1,3,4-oxadiazole derivatives in great incomes [4,5].
In addition, condensation of hydrazide derivatives with carboxylic acids followed by dehydrative cyclization give these important oxadiazole derivatives [6]. Microwave acceleration of oxadiazole derivatives synthesis has appeared as an appreciated alternative to conventional approaches in latest periods. These synthesis reactions are less period consuming and high yielding [7] and [8]. It is well-known that substituted-1,3,4-oxadiazole can be used as synthons for others biologically important compounds.So, it was of interest to investigate aspects of synthesis new series of Novel 2,5-disubstituted-1,3,4-Oxadiazoles.
Experimentat Section
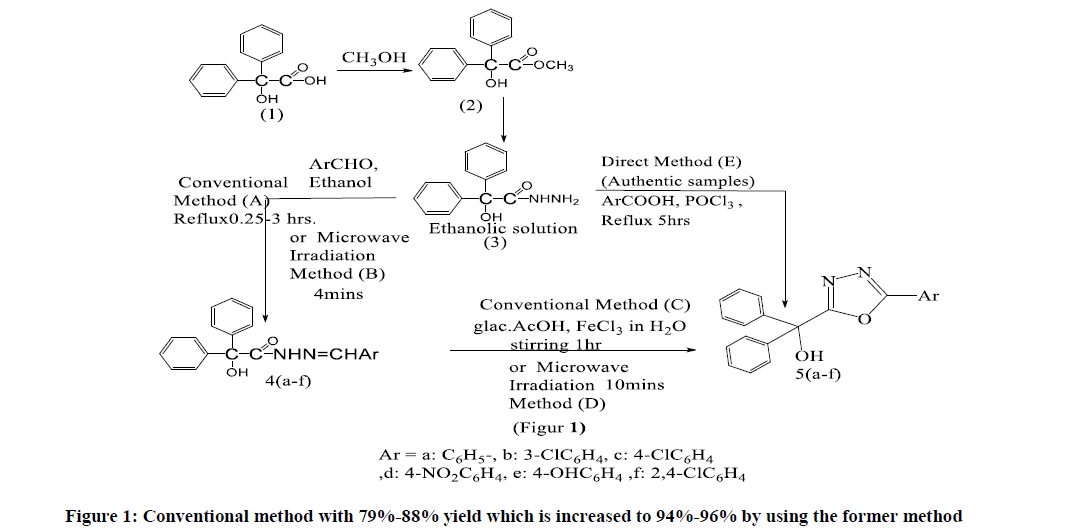
Melting points were measured using electro thermal 9300 melting point appliance and are uncorrected. IR spectra were recorded on a Perkin-Elmer 590 B spectrophotometer using KBr disk. UV spectra were recorded on Shimadzu UV-160 spectrophotometer using methanol as a solvent 1H-NMR and 13C-NMR spectra were recorded on a Bruker 400 MHz spectrometer in DMSO-d6 with TMS as the internal standard. Elemental analysis was performed on Carlo-Erba 1160 CHN analysis. All reagents and solvents were obtained from commercial suppliers and were used without further purification; the benzilic acid hydrazide was prepared by author [9], thus. The ester of benzylic acid (2) was prepared by the usual esterification method, benzilic acid hydrazide (3) was synthesized using reported method starting from ester (methyl benzilate) [9].
Synthesis of Substituted Benzilyl Hydrazones (4a-f) Conventional Method (A)
To a hot ethanolic solution of benzilic acid hydrazide (3) (0.24 gm, 1 m mole), a solution of appropriate aromatic aldehydes (1 m mole) in 20 ml of methanol is added and the reaction mixture is refluxed for (0.25-3 hrs.).On cooling the resulting product is filtered, dried and recrystallized from ethanol to give the substituted hydrazones (4a-f). Physical and spectral data are recorded in Tables 1 and 2 [10].
| Comp. No. | Ar | Reaction time (hrs.) | m.p. C̊ | Yield% conventional (MW) | Molecular formula | CHN Analysis% found (Calcd.) | ||
|---|---|---|---|---|---|---|---|---|
| C% | H% | N% | ||||||
| 4a | C6H5- | 2 | 200-202 | 79 (96) | C21H18N2O2 | 75.98 | 5.55 | 8.29 |
| (76.36 | 5.45 | 8.48) | ||||||
| 4b | 3-ClC6H4 | 0.5 | 216-218 | 80 (94) | C22H17 C |
69.0 | 4.61 | 7.49 |
| (69.13 | 4.66 | 7.68) | ||||||
| 4c | 4-ClC6H4 | 0.25 | 248-249 | 85 (94) | C22H17 ClN2O2 | 69.96 | 4.62 | 7.52 |
| (69.13 | 4.66 | 7.68) | ||||||
| 4d | 4-NO2C6H4 | 0.5 | 266-268 | 91 (97) | C21H17N3O4 | 66.88 | 4.61 | 10.93 |
| (67.02 | 4.53 | 11.02) | ||||||
| 4e | 4-HOC6H4 | 1 | 253-255 | 81 (95) | C21H18N2O3 | 72.64 | 5.31 | 8.00 |
| (72.83 | 5.20 | 8.09) | ||||||
| 4f | 2,4-ClC6H4 | 1 | 235-237 | 88 (94) | C21H16Cl2N2O2 | 63.19 | 3.85 | 6.86 |
| (63.15 | 4.01 | 7.01 ) | ||||||
Table 1: Physical data for synthesized compounds (4a-f)
Microwave Irradiation Method (B)
An appropriate aromatic aldehyde (1 m mole) is added to completely dissolved benzilic acid hydrazide (3) (0.24 gm, 1 m mole) in 12 ml. ethanol. The reaction mixture is kept under microwave irradiation for 4 minutes at medium-low temperature. After completing the reaction (from the checked progress of reaction using thin layer chromatography spotting in dichloromethane as eluting solvent, immediately added the result mixture to cold-water bath. Orange yellow-colored crystals of compounds (4a-f) are recrystallized from methanol. % Yields are recorded in Table 1 [11].
Preparation of 2-Substituted-5-(α, α-Diphenyl-α-Hydroxymethyl)-1,3,4-Oxadiazoles (5a-f)
Conventional method (C): A mixture of one of benzilyl hydrazones(3) (4a-f) (0.001 mole) in dioxane (25 ml), a solution of ferric chloride (10 mg) in water and glacial acetic acid (10 ml) is stirred for one hour, diluted with water (200 ml) and kept at room temperature for 72 hrs. The product is filtered and crystallized from ethanol to give the matching 2,5-disubstituted-1,3,4-oxadizoles(5a-f).into ice-water and made basic by adding solution of sodium bicarbonate. The resulting solid is filtered, dried and crystallized from aqueous ethanol to give the title oxadizoles (5a-f). Physical and spectral data are recorded in Tables 2 and 4 [12].
| Comp. No. | Ar | Method | m.p. C̊ | Yield% conventional (MW) | Molecular formula | CHN analysis% found (Calcd.) | ||
|---|---|---|---|---|---|---|---|---|
| C% | H% | N% | ||||||
| 5a | C6H5- | A | 221-223 | 51 (95) | C21H16N2O2 | 76.69 | 4.71 | 8.55 |
| (76.82 | 4.87 | 8.53) | ||||||
| 5b | 3-ClC6H4 | A,B | 232-234 | 52 (91) | C21H15 ClN2O2 | 69.83 | 4.02 | 7.63 |
| (69.51 | 4.13 | 7.72) | ||||||
| 5c | 4-ClC6H4 | A,B | 213-215 | 68 (91) | C21H15 ClN2O2 | 69.44 | 4.16 | 7.55 |
| (69.51 | 4,13 | 7.72) | ||||||
| 5d | 4-NO2C6H4 | A,B | 191-193 | 67 (93) | C21H15N2O3 | 67.41 | 3.88 | 11.17 |
| (67.56 | 4.02 | 11.26) | ||||||
| 5e | 4-OHC6H4 | A | 187-189 | 47 (92) | C21H16N3O4 | 73.29 | 4.29 | 8.19 |
| (73.25 | 4.65 | 8.13) | ||||||
| 5f | 2,4-ClC6H4 | A.B | 194-195 | 44 (97) | C21H14 Cl2N2O2 | 63.22 | 3.44 | 6.82 |
| (63.47 | 3.52 | 7.05) | ||||||
Table 2: Physical data for synthesized compounds (5a-f)
Microwave irradiation method (D): A mixture of one of compounds (4a-f) (0.001 mol) and 0.5 ml of acetic acid is irradiated in a microwave phial (30 ml) at 130 °C for 10 min. After completion of the reaction (monitored by thin layer chromatography, ethyl acetate/hexane, (5:2), the product is workup as method (C). % Yields are listed in Table 2 [13].
Direct method (E); (Authentic samples): A mixture of benzilic acid hydrazide (3) (0.24 gm, 0.01 mole), appropriate aromatic carboxylic acids (0.01 mole) and phosphorus oxychloride (5 ml) are refluxed for five hours. After cooling, the reaction mixture is poured into ice-water and made basic by adding solution of sodium bicarbonate. The resulting product is filtered, dried and crystallized from aqueous ethanol to give the title – oxadizoles (5a-f). Physical data and spectral data are recorded in Table 2 and 4 [2,14].
Results and Discussion
All methods for synthesis of the target compounds is accomplished by the routes outlined in Schemes (1). Benzilic acid hydrazide reacts with suitable aromatic aldehydes to give the corresponding hydrazones (4a-f), Scheme (1). This reaction is carried out using two methods; the primer is method (A), a conventional method with 79%-88% yield which is increased to 94%-96% by using the former method in Figure 1 (B) with microwave irradiation (only 10 minutes) in Figure 2.
UV, IR and 1H-NMR spectral data (Table 3), identified the structure of these hydrazones. UV spectrum shows λmax (CH3OH) at (269-277 nm) due to the conjugation that cause bathochromic shift, Scheme (2).
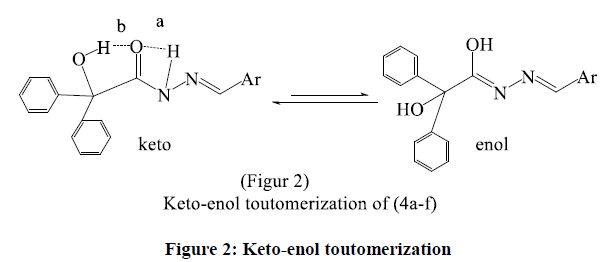
Ketone form is expected in this paper to be more stable due to it is stabilized by an internal hydrogen bond with amine proton (b) or benzylic hydroxyl proton (b ) From the computational study, the total energy of keto form is (- 4.4824), while that of enol form is (5.3617 kcal/mole. This indicates that the keto form is more stable by 9.8441 kcal/mole, and it is the main tautomeric form is the ketone.IR (cm-1) spectra of these hydrazones, (Table 3): 3285- 3380(NH), (1635-1664 cm-1) (C=O) and (1570-1593) (C=N). These results come with agreement of the published ones [15]. These low absorptions of carbonyl also due to the conjugation, which decrease the double bond property, the actual position of the C=O bond is affected by several factors; one of them is conjugation [16]. Conventional method (C) is the oxidative cyclization of benzilyl hydrazones (4a-f) to the target compounds (5a-f), which is carried out by stirring these hydrazones for one hour with glac, acetic acid and aq. ferric chloride solution, or method (D) with microwave irradiation for ten minutes.
| Comp. Number | IR(KBr) γ cm-1 | UV λmax (nm) MeOH | 1 H-NMR δ (ppm) DMSO-d6 | 13C-NMR δ (ppm) DMSO-d6 | ||
|---|---|---|---|---|---|---|
| C=N | C=O | NH | ||||
| 4a | 1586 | 1660 | 3335 | 269 | 6.3(s,1H,OH);6.8-7.2(m,15H,3 Ph);8.1(s,1H,CH);10.2(s,1H,NH) | 101.2, 126.3, 128.4, 129.3, 131.3, 133.6, 142.4, 143.3. |
| 4b | 1586 | 1642 | 3375 | 270 | 6.6(s,1H,OH);6.9(m,10H,2Ph);7.3-7.6(m,4H,ArH);8.1(s,1H,CH);10.1(s,1H,NH). | (101.4,125.8,127.4,128.5,Ph);8.1(s,1H, CH);10.2(s,1H,NH) 129.4,130.5,131.7,134.5,133.8,131.5142.8,143.6. |
| 4c | 1592 | 1635 | 3362 | 271 | 6.4(s,1H,OH);6.8-7.2(m,10H,2Ph);7.7(m,4H,ArH);8.4(s,1H,CH);10.1(s,1H,NH). | 101.1,125.5,127.6,128.3,129.8,130.5,131.4,134.3,133.4,131.5,142.2,143.5. |
| 4d | 1570 | 1635 | 3380 | 274 | 6.3(s,1H,OH);7 (m,15H,2Ph);7.1-7.5(m,4H,ArH).8.1(s,1H,CH);10.4(s,1H,NH) | 101.7,124,2,124.6,126.6,127.7,128.2,129.3,139.1,142.4,143.8,160.2. |
| 4e | 1590 | 1635 | 3368 | 278 | 5.7(s,1H,phenolicOH),6.4(s,H,OH);6.7-7.3(m,10H,2Ph);7.47.8(m,4H,ArH);8.3(s,1H,CH);10.0(s,1H,NH). | 101.4,116.7,126.3,128.8,129.4,129.7,130.4,142.9,143.6,160.1. |
| 4f | 1595 | 1664 | 3285 | 277 | 6.4(s,1H,OH);6.5-7.2(m,10H,2Ph);7.3-7.6(m,3H,ArH);8.1(s,1H,CH);10.2(s,1H,NH). | 101.7,126.2,128.2,129.3,130.2,131.2,142.45,143.7,160.6 |
Table 3: Spectral data for synthesized compounds (4a-F)
Finally, a preliminary study was conducted by which the insecticidal and antifungal activities of the synthesized hydrazones were in vitro bioassays showed that these compounds have moderate fungicidal activity against fungus mycelium growth. Furthermore, hydrazones (4a and 4e) are a promising significant fungicide for further development. While oxadiazoles (5h and 5f) showed good insecticidal effect on larvae of kapra beetle and the adults of rose aphids (Table 4).
| Co. No. | IR(KBr) γ cm-1 | UV λmax (nm) | 1 H-NMR δ (ppm) DMSO-d6 | 13C-NMR δ (ppm) DMSO-d6 | |
|---|---|---|---|---|---|
| C=N | C-O-C | ||||
| 5a | 1640 | 1245 | 241 | 6.4(s, 1H, OH) ;6.6-7.4(m, 15H, 3Ph). | 91.3,121.9,126.1,127.5,127.8,128.2,129.8,130.1,131.7,143,5,163.3,164.8. |
| 5b | 1645 | 1248 | 243 | 6.2(s, 1H, OH) ;7.1 (m, 10H, 2Ph) ;7.2-7.5(m3H, ArH). | 91.6,122.4,125.3,126.3,127.3,128.2,129.3,134.7,143.6,164.8. |
| 5c | 1650 | 1255 | 248 | 6.2(s, 1H, OH) ;7.0 (m, 10H, 2Ph) ;7.2-7.5(m3H, ArH). | 91.5,122.2,125.2,126.3,127.6,128.2,129.3,134.4,143.3,164.5. |
| 5d | 1633 | 1250 | 252 | 6.5(s, 1H, OH) ;7.1 (m, 10H, 2Ph) ;7.1-7.6(m3H, ArH). | 91.6,126.2,128.2,128.4,129.1,129.6,130.4,132.5,143.8,148.2,163.6. |
| 5e | 1640 | 1238 | 250 | 6.1(s, 1H, OH) ;6.5-6.9(m, 10H, 2Ph) ;7.4-7.8(m3H, ArH). | 91.4,116.3,116.8,118.8,126.5,128.2,128.7, 129.5,143.4,158.3,163.2,164.5. |
| 5f | 1632 | 1246 | 247 | 6.3(s, 1H, OH) ;6.7-7.2(m, 10H, 2Ph) ;7.3-7.8(m3H, ArH). | 90.9,126.2,127.5,128.5,129.6,130.2,133,6,135.5,135.8,143.4,163.5,166.1. |
Table 4: Spectral data for synthesized compounds (5a-f)
Conclusion
From the experiment it was concluded that both the methods could be used for production of benzilyl hydrazones (4a-f) and oxadizoles (5a-f) but microwave irradiation has several advantages over conventional one. Microwave energy is additional effective revenue of cooking reactions. Chemical conversions that procured periods to complete can now be accomplished in few minutes. Microwave energy offers many assistances for carrying out preparation including improved reaction rates, income enhancements, and chemically cleaner. Finally, these hydrazones have moderate fungicidal activity against fungus mycelium growth, while oxadizoles showed good insecticidal effect.
Acknowledgments
The authors acknowledge and thank the Faculty of Science, Department of Chemistry, University of Mosul for their support and support to complete this research. The authors are also thankful to staff of Faculty of Science, Department of biology, University of Mosul for the measure the biological activity for the prepared compounds.
References
- A Kumar, SS D’Souza, SR Nagaraj. Cancer Chemother Pharmacol. 2009; 64(6), 1221–33
- MA Martinez-Grau, ICG Valcarcel, et al. Bioorg Med Chem Lett. 2018; 28(10), 1758–64.
- RM Shingare, YS Patil, JN Sangshetti. Med Chem Res. 2018; 27(4), 1283–91.
- JL Zheng. Synlett. 2017; 28(11), 1373–7.
- Q Gao, S Liu, X Wu. Org Lett. 2015; 17(12), 2960–3.
- K Tokumaru, JN Johnston. Chem Sci. 2017; 8(4), 3187–91.
- S Caddick. Tetrahedron. 1995; 51(38), 10403–32.
- NA Shafakat, B Dar, V Pradhan. Mini Rev Med Chem. 2013; 13(12):1792–800.
- BA Ahmed, SJ Mohammed, BT Khalil. Rafidain J Sci. 2014; 25(1E):62–8.
- SJ Mohammed, FG Zuhair.
- S Guin, T Ghosh, SK Rout. Org Lett. 2011; 13(22), 5976–9.
- OO Ajani, KT Iyaye, OY Audu, SJ Olorunshola. J Heterocycl Chem. 2018; 55(1), 302–12.
- GVS Kumar, Y Rajendraprasad, BP Mallikarjuna. Eur J Med Chem. 2010; 45(5), 2063–74.
- KM Khan, M Rani, S Perveen. Lett Org Chem. 2004; 1(1), 50–2.
- P Singh, PK Sharma, JK Sharma. Org Med Chem Lett. 2012; 2(1), 8.
- A Mahal, R Abu-El-Halawa, SA Zabin. World J Org Chem. 2015; 3(1), 1–8.