Original Articles: 2024 Vol: 16 Issue: 6
Doped Zno Nanostructures by Polyol Method with Antibacterial Properties
A. Ferreiro1, C. Martín2, M.E. Rabanal1*
1Department of Material Science and Chemical Engineering, Avenida de la Universidad 30, Leganés, Spain
2Departmentof Bioengineering, Avenida de la Universidad 30, Leganés, Spain
- Corresponding Author:
- M.E. Rabanal
Department of Material Science and Chemical Engineering, Avenida de la Universidad 30, Leganés, Spain
Received: 31-Dec-2023, Manuscript No. JOCPR-23-121407; Editor assigned: 04-Jan-2024, PreQC No. JOCPR- 23-121407 (PQ); Reviewed: 18-Jan-2023, QC No. JOCPR-23-121407; Revised: 25-Jan-2023, Manuscript No. JOCPR-23-121407 (R); Published: 01-Feb-2024, DOI:10.37532/0975-7384.2024.16(2).109.
Citation: Rabanal ME, et al. 2024. Doped Zno Nanostructures by Polyol Method with Antibacterial Properties. J. Chem Pharm.Res. 16:109.
Copyright: © 2024 Rabanal ME et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The growing expansion of multidrug-resistant organisms due to the abundant use of antibiotics is increasing the need to develop alternative antibacterial methods. Zinc Oxide (ZnO) systems are very promising materials in this field. Here, the synthesis of undoped and co-doped ZnO nanoparticles, morphological and structural characterization using Scanning Electron Microscope (SEM) and X-Ray Diffraction (XRD) techniques were carried out. ZnO systems can interact physically (mainly with the breakdown of cell envelope) or chemically (mainly with the generation of Reactive Oxygen Species (ROS)) within living organisms to perform their antibacterial action. Well-diffusion, Disk diffusion and Colony forming unitsâ?? techniques, were used and the antibacterial tendency were examined. The findings in this research suggest that both, physical and chemical antibacterial interactions of the ZnO systems may be occurring, making these nanomaterials promising for potential use in antibacterial coatings.
Keywords
Nanoparticles, Doped ZnO, ROS, Antibacterial properties, E.coli.
Introduction
Since the discovery of penicillin almost a century ago [1], the excessive use of antibiotics has led to the emergence and proliferation of multidrug-resistant organisms. In recent years, the rise of multidrug-resistant organisms has evolvedfrom being solely a clinical issue to becoming a global threat to many countries' development and economies [2].
Nanotechnology has significantly broadened the possibilities in the development of materials with antibacterial properties. Among various types of nanoparticles, metallic nanoparticles, particularly silver and zinc oxide nanoparticles, have garnered considerable attention. Zinc oxide nanoparticles have applications in the food, medical, pharmaceutical, and cosmetic industries [3]. Various methods exist for their synthesis, with a key challenge being the identification of the most cost-effective and straightforward approach. In this research, the polyol method [4] was selected as the synthesis technique.
Zinc oxide is a n-type semiconductor (II-VI group) [5,6]. This material is particularly appealing in numerous fields because it possesses a broad energy band gap (3.37 eV) [7], large exciton binding energy (60 meV), and high thermal and mechanical stability at Room Temperature (RT). The wide band gap of ZnO has various effects on its properties, includingits electrical conductivity and optical absorption. Furthermore, ZnO is known for its biocompatibility, biodegradability, and low toxicity [8]. It exhibits three distinct crystalline structures: Cubic zinc blende, cubic rocksalt, and hexagonal wurtzite. Among these, the wurtzite structure is the most prevalent [9], offering superior stability under ambient conditions. The wurtzite structure features a hexagonal unit cell and belongs to the P63mc space group.
As mentioned previously, ZnO is classified as a semiconducting material. To enhance its conductivity and further improve its properties, ZnO is often doped with various elements [10]. Doping ZnO offers the possibility to alter the size of nanoparticles and increase the production of Reactive Oxygen Species (ROS), thereby achieving a significantly enhanced antibacterial effect [11-13]. Li can be incorporated into the structure of ZnO systems. The presence of Li leads to a modification of the energy gap in ZnO nanoparticles and promotes the production of ROS [14-15], which is one of the most notable mechanisms associated with the antibacterial action.
The incorporation of Rare Earth (RE) elements into ZnO [16] has been the focus of numerous studies enhanced conductivity, photoluminescence, and photoelectrochemical properties [17-19]. It has been demonstrated that introducing Nd into ZnO significantly amplifies the photocatalytic activity of the nanoparticles. Furthermore, doping the nanoparticles with RE elements affects the growth of nanoparticles, resulting in smaller-sized nanoparticles due to the incorporation of RE within the nanoparticle's structure. Doping with RE elements also induces a modification of the band gap, as mentioned earlier, leading to an increased production of Reactive Oxygen Species (ROS) [20]. The unique 4f configuration of RE elements reduces the recombination time of the electron/hole pair in the semiconductor [21], resulting in a more efficient photocatalytic process and, consequently, heightened antibacterial activity [22].
ZnO is a material used in the biological field [23], due to its low toxicity, stability and efficient biological properties. Besides it shows significant bactericidal properties over a broad range of Gram-positive and Gram-negative bacteria such as Escherichia coli, S. enteritidis, Streptococcus pyogenes, Aeromonas hydrophila, B. subtilis, S. aureus, L. monocytogenes, Klebsiella pneumonia, P. aeruginosa, Salmonella typhimurium, E. faecalis, etc. [24-29].
At the nanoscale, the particles possess a considerably larger surface area than the macro-scale, resulting in a higher percentage of atoms on the surface. These free atoms on the surface are considerably more reactive when interacting with the elements in their surroundings. In summary [30], the increased surface area and other characteristics resulting from their nanostructure enable nanoparticles to readily interact with biomolecules in the environment, facilitating their penetration into the internal structures of cells.
Recently, it has been demonstrated that polyol synthesis is one of the most effective methods for producing metal oxide nanoparticles within the size range of 10 to 100 nm [31]. Nucleation is the primary mechanism for this type of synthesis. Through the utilization of various high-boiling-point polyols, including ethylene glycol, diethylene glycol, tetraethylene glycol, or glycerol, it becomes feasible to regulate the growth of nanoparticles and prevent their agglomeration [32]. Moreover, it is a straightforward and expeditious method that does not necessitate highly specialized equipment.
According to the literature, key findings from studies on ZnO nanoparticles include: ZnO nanoparticles exhibit effectiveness against a diverse range of bacteria, including both Gram-positive and Gram-negative strains [33]. Besides, the antibacterial activity of ZnO nanoparticles tends to improve with decreasing particle size [34], resulting in a larger surface area. Many authors report that a higher concentration of the nanoparticle solution leads to a more pronounced antibacterial effect. Various mechanisms underpin the antibacterial properties of nanoparticles [35-36], although they are not yet fully comprehended. Among these mechanisms, the generation of Reactive Oxygen Species (ROS) stands out as one of the most significant [37-39]. This research work is focused to exploring the behavior of emerging doped ZnO nanoparticles with rare earth and alkali elements for potential use in antibacterial coatings.
Materials and Methods
Materials
The Commercial Zinc Oxide (Sigma-Aldrich, 99%) was employed as reference to compare the behavior with samples synthesized. Nanoparticles of codoped ZnO /Li/Nd were prepared by polyol method using reagents of high purity, such as zinc acetate dihydrate, (Zn(Ac)2•2H2O) (Sigma-Aldrich, 99%), neodymium nitrate hexahydrate, (Nd(NO3)3•6H2O), (Sigma Aldrich, 99%), and lithium acetate (LiCH3CO2), (Sigma Aldrich, 99%). The solvent was Ethylene Glycol (EG). Poly Vinyl Pyrrolidone (PVP) as capping agent were used. In the biological experiments, the materials: Luria Broth Base (Miller’s LB Broth Base), LB agar, Miller (Agar), Escherichia coli strain B, (E.coli), Hydrogen peroxide (H2O2) (Sigma-Aldrich) were used.
Experimental procedure
Synthesis of the nanoparticles by polyol method
Codoped and pure zinc oxide nanoparticles were synthesized using polyol method with the respective stoichiometric amounts of reagents to obtain the desired nominal stoichiometry [40]. In a three-neck flask, the starting reagents were dissolved in 30 mL of EG as solvent and continuously stirred and heated, forming a colorless solution. When the temperature reaches 90°C, five mmol PVP previously dissolved in 20 mL of EG is added, and the solution is further heated until an evident white color appears (160°C-180ºC), then, one mL of distilled water is added, and the reaction is maintained for 30 min. at a fixed temperature of 180ºC. Finally, a slow cooling under constant stirring is carried out. The white precipitate obtained is separated by centrifugation (6000 rpm) and subjected to successive washings, initially with methanol and later with distilled water. The white powders are then dried in an oven at 90°C for 12 h in an ambient atmosphere. A post thermal treatment at 500°C for three h to increase the crystallinity of as prepared samples has been carried out.
In order to test the antibacterial effect of the ZnO systems, three different kind of samples were studied as indicated in (Table 1).
| Sample | Nominal composition | Label |
|---|---|---|
| Commercial Zinc Oxide | Pure commercial ZnO | ZO-com |
| Undoped Zinc Oxide nanoparticles | Pure ZnO | ZO-NP |
| Codoped Zinc Oxide nanoparticles | ZnONd (1%at.) Li (0.5%at.) | ZONLi-NP |
Table 1: Label, nominal composition for samples.
Characterization techniques:
Samples The crystallinity and the identification of the phases present were analyzed by X-Ray Powder Diffraction (XRD) using Philips XPert 30XL with the Cu Kα radiation λ=1.5406 Å in the 2θ range between 20° and 75° with a time step 0.02.
A Scanning Electron Microscope (SEM) (FEI Teneo) with EDS (Energy Dispersive Spectroscopy) detector has been used to study the morphology and composition of the nanoparticles. Image J software was used to measure the size of Nps.
Biological evaluation
Disk diffusion method:
This test was performed with bacterial suspension using LB agar. UV sterilized filter paper disks of 8 mm diameter were set on the agar, and a colloidal suspension with 10 μL of the NPs in distilled water at different concentrations was soaked on them. Besides, in each Petri dish, a negative control of H2O2 (1M) was added to ensure that the experiment was correctly performed and the antibacterial effect was possible [41-42].
A stock solution of nanoparticles with a concentration of 10 mg/mL in distilled water was diluted for preparing the final suspensions of nanoparticles. The final concentrations and volumes of nanoparticles suspensions and distilled water are shown in (Table 2).
| Concentration (mg/mL) | Nanoparticles (µL) | H2O (µL) |
|---|---|---|
| 1.0 | 10.0 | 90.0 |
| 3.0 | 30.0 | 70.0 |
| 5.0 | 50.0 | 50.0 |
Table 2: Amount of components for each concentration in the well diffusion method.
| Concentration (mg/mL) | Bacteria (µL) | PBS (µL) |
|---|---|---|
| Control | 0 | 400.0 |
| 1.0 | 50.0 | 350.0 |
| 3.0 | 150.0 | 250.0 |
| 5.0 | 250.0 | 150.0 |
Table 3: Amount of components for each concentration for 100 µL bacterial suspension in the colony forming technique.
Well diffusion method:
The colloidal suspensions (30 μL) were soaked inside of the 8 mm diameter holes. The amount of each components forming the suspension at different concentration is listed in Table 2. In each of the Petri dishes, two controls were added: One with H2O2 (1M) and another with distilled water.
Colony-forming units’ measurements:
One Aliquots of E.coli bacteria were tempered by adding LB medium and incubated at 37ºC. After this process, the Optical Density (O.D) of the samples was measured over 600 nm using a Biowave II spectrophotometer (Biochrom, UK). The cells were left growing until they reached a concentration of 2.9 x 106 CFU/mL.
Then, the cells were centrifuged at 13000 rpm for 10 min and the supernatant was discharged. Then, the pellet was washed with PBS two more times to remove any trace of LB and the cells were transferred to a Falcon tube with 0.5 mL of PBS. Finally, the incubation of E.coli in PBS at 200 rpm and 37ºC for 2h in the presence of the samples was performed. The dispersion was added to the corresponding Falcon tubes containing the bacteria in different amounts depending on the desired concentration. All these steps were performed in the vicinity of a flame to avoid sample contamination. Amount of components for each concentration are shown in (Table 3).
Finally, after preparing dilutions, each solution was seeded on Petri dishes and incubated overnight at 37ºC. Following all the experiments, photos were taken and the data analysis were carried out. The graphs represented the E.coli viablity (%) versus the concentration of the colloidal suspension (mg/mL). All the treatments were performed at least in triplicate.
In order to calculate the cell viability (%), the following formula was used:
Viability (%) = CFUsamplex100/CFUcontrol (Eq…1)
Results and Discussion
Morphological analysis:
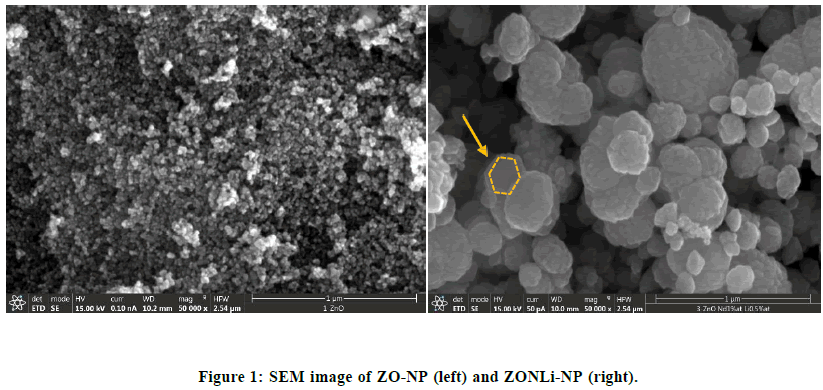
ZO-com is a bulk material composed of particles with a macro-scale size. SEM analysis was performed on both doped ZnO nanoparticle compositions: ZO-NP and ZONLi-NP. In Figure 1 (left) it is shown the SEM image (50000x) for ZO- NP. It can be clearly seen that the nanoparticles have a spherical shape and they are compacted among them [43]. The average diameter of nanoparticles is 21 ± 5 nm and the surface area (BET) is 6.5 (m2/g).
Similar in Figure 1 (right), show the SEM image (50000x) of ZONLi-NP. These nanoparticles have a quasi-spherical shape, similar to ZO-NP, but in this case, they exhibit larger agglomarates (formed by a stack of quasi-hexagonal sheets) with the average diameter of 190 ± 60 nm and a surface area is 23.9 (m2/g), nanoparticles with hexagonal morphologies (platelike) probably to show higher ROS due to a higher proportion of surfaces/polar faces [44]. In literature, authors report that codoped samples with multi-atomic centers could be the origin of active sites for O2 adsorption and the cause of higher ROS production [45]. An increase in surface area provides more active sites and interaction sites, therefore the production of ROS may enhance the antibacterial effect, moreover mechanical interactions between the greater nanoparticles and the bacteria, leading to mechanical damage.
Structural Analysis:
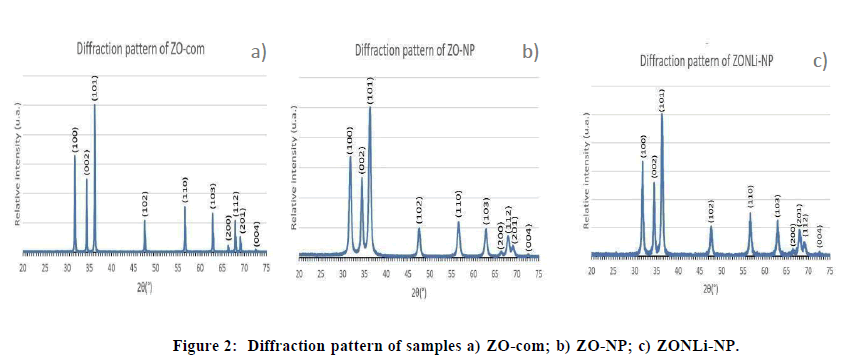
X- Ray Diffraction technique was used to determine the crystalline structure of the prepared samples and compare them. In (Figure 2A) shows the diffraction pattern of ZO-com, the diffraction peaks in the diffractogram correspond to the hexagonal wurtzite phase [46]. ZO-NP in (Figure 2B) shows there is no presence of additional peaks in the diffraction pattern compared with pure ZnO, so there are not secondary phases to identify. On the other hand, no additional peaks were observed in the diffraction pattern of ZONLi-NP (Figure 2C), Li and Nd cations were incorporated into the wurtzite structure of the ZnO system. A substitution occurred where Zn was replaced by Li and/or Nd. A slight shift to higher angles is appreciated causing a decrease in lattice parameters, due to differences in ionic radius Zn2+ (0.60 Å), Nd3+ (0.98Å), Li1+ (0.59Å), and valence of ions respecting to Zn ion, it resulted in internal changes on the original structure, modifying the ideal wurtzite structure [47-49]. Doping with Nd and Li produces broadening and low intensity of the peaks contributing to low crystallinity and small crystallite size. At the micro scale, there is greater crystallinity due to reduced surface effects, larger crystals may have a more ordered structure and less influence from surface defects than nanocrystals. This may explain the larger crystallite size of commercial zinc oxide (Figure 2).
XRD data were calculated in accordance with bibliography [50], the lattice parameters, the unit cell volume, Crystallite Size (CS), dislocation density (δ), Zn-O bond length (L), lattice strain (ε), positional parameter (u), and Atomic Packing Fraction (APF) for each sample were represented as shown in the (Table 4).
| Sample | a=b (Å) | c (Å) | Unit cell Volume (Å) | CS (nm) |
δ x (10-3)(nm-2) | L(Å) | ε (W-H)x (10-3) | u | APF (%) |
|---|---|---|---|---|---|---|---|---|---|
| ZO(JCPDS: 891397) | 3.253 | 5.213 | 47.8 | ||||||
| ZO-com | 3.249 | 5.212 | 47.6 | 68 | 0.22 | 1.978 | 0.556 | 0.37953 | 75.38 |
| ZO-NP | 3.247 | 5.211 | 47.6 | 17 | 3.46 | 1.977 | -2.83 | 0.37942 | 75.35 |
| ZONLi-NP | 3.23 | 5.204 | 47.1 | 22 | 2.07 | 1.969 | -4.008 | 0.37841 | 75.05 |
Table 4: Estimated structural parameters from XRD analysis data of the samples.
Both ZO-com and ZO-NP exhibit values for a,b,c, and unit cell volumen that closely resemble those of ZO (JCPDS card no: 891397), which represents reference values found in the literature [51]. It has been observed that the value of u decreases slightly in such a way that the four tetrahedral distances almost remain nearly constant by distorting tetrahedral angles. Besides, the Zn–O bond length for nanoparticles samples and APF behavior with doping are agreement with literature [50].
However, the parameters for ZONLi-NP show more significant deviations from reference values, a decrease in cell volumen could be explained either by a decrease in the O–Nd bond length or to an increase in Zn vacancies for charge compensation. Moreover, this difference is attributed to the lattice strain (applying Williamson-Hall analysis), compressive stress is observed for the two samples, more accentuated by the introduction of Nd and Li cations into the wurtzite structure [40,52-53].
The antibacterial properties
Well diffusion method
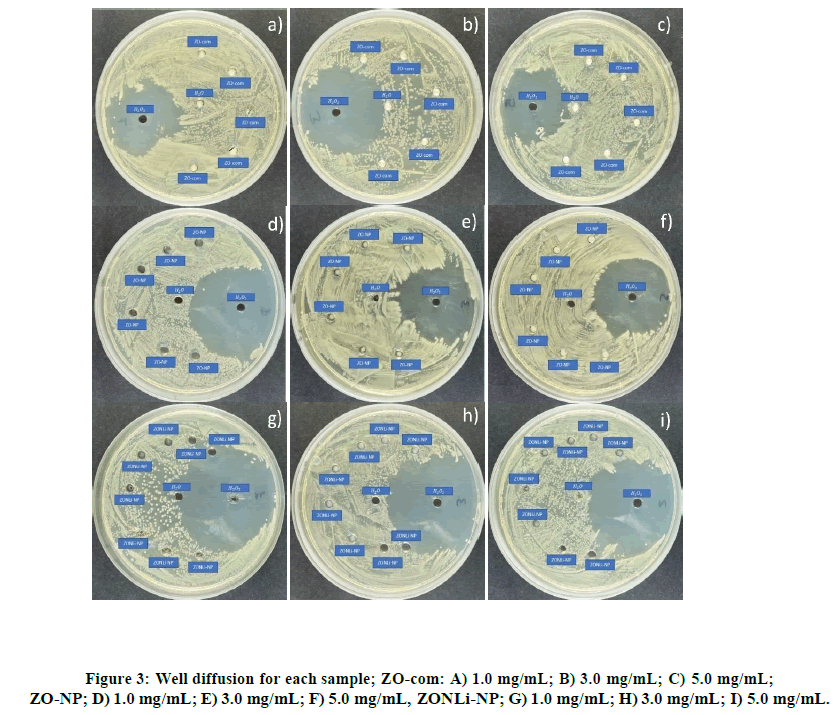
As shown in Table 2, the well diffusion method [54-55] was performed at three different concentrations: 1.0, 3.0, and 5.0 mg/mL using commercial ZnO and the two compositions of ZnO NPs. In each plate displayed in Figure 3, there is a negative control with H2O2, a control with H2O, and pure commercial ZnO suspensions at varying concentrations, (Figures 3A-3C). No inhibition zone was observed in any cases, possibly due to insufficient contact between the bacteriaand the ZnO system. Similar behavior is appreciated for ZO-NP (Figures 3D-3F), there is no bacterial growth, it could be observed maybe due to the lack of physical and chemical contact. In (Figures 3G-3I) there are two controls and replicas of ZONLi- NP, as in the two previous cases, no inhibition zone could be observed. In summary, no bacterial killing could be observed in any of the cases due to the low contact between the bacteria and the ZnO systems. In all cases, it could be observed how the nanoparticles were deposited on the bottom of the plate instead of diffusing through the agar provoking a possible lack of physical contact and/or avoiding the diffusion of ROS.
Disk diffusion method
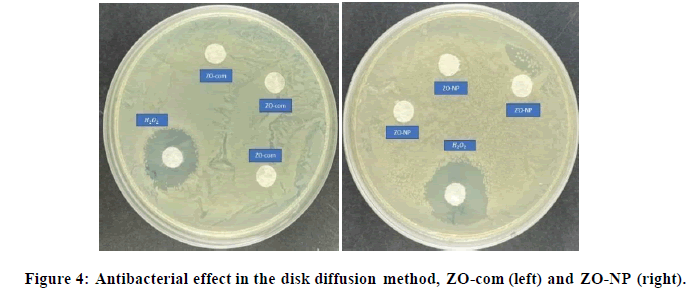
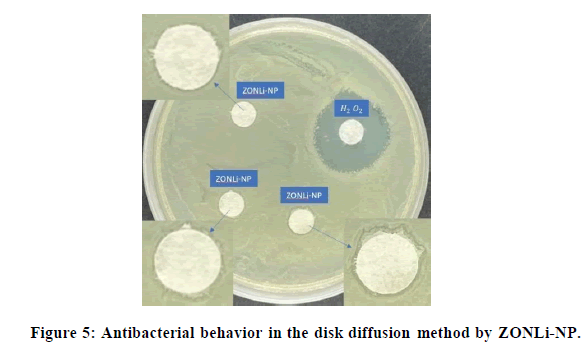
This experiment [56-57] is quite similar to the previous one, however, with this, the deposition in the wells was tried to be avoided. As can be shown in Figure 4, in the ZO-com and ZO-NP, no halo could be appreciated around the filter paper discs. The antibacterial behavior for H2O2 control observed when a big halo around it in both cases, however, there was no zone of inhibition produced by the ZnO systems. An explanation for the lack of bactericidal effect could be that the samples were getting trapped inside the filter paper avoiding the correct diffusion of ROS and the physical contact. Nevertheless, the results were different for the ZONLi-NP.
In the case of ZONLi-NP, in the amplified images in Figure 5, a halo could be appreciated. The halo presence in this case could be explained by doping with RE elements, modifying the band gap and increasing ROS production, thereby enhancing the antibacterial effect.
The presence of filter paper discs may have prevented direct contact between the ZnO system and the bacteria in the cases of ZO-com and ZO-NP. As a result, physical interactions such as the mechanical rupture of the bacterial membrane may not have occurred. However, in the case of ZONLi-NP, due to the increased production of ROS resulting from alterations in the valence band in the presence of visible light, a chemical interaction could have occurred, leading to the observed zone of inhibition. This led us to conclude that ROS diffusion was one of the mechanisms at play.
Considering these findings, we decided to explore a new method in which there was no doubt that the ZnO samples were in close contact with the bacteria. This was done to further confirm that physical interactions are indeed an antibacterial mechanism against E.coli.
Colony-forming unit method:
In the two preceding experiments was challenging to ensure direct contact between the nanoparticles and the bacteria due to their static nature. However, the colony-forming unit technique [58], is a dynamic method that guarantees such contact. Through this method, we were able to confirm the presence of physical mechanisms, in addition to the observed ROS diffusion.
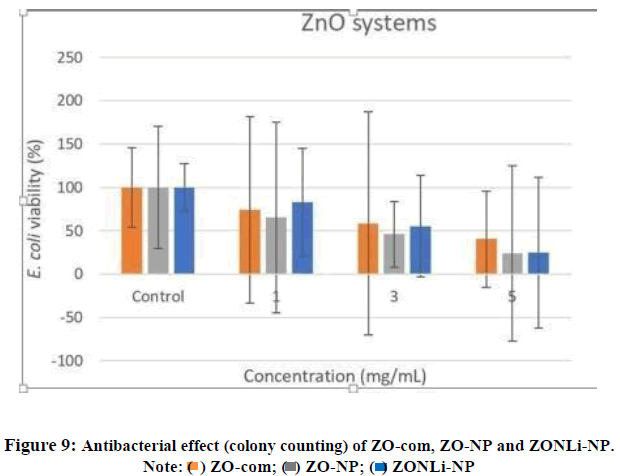
The bactericidal tests in ZO-com, ZO-NP and ZONLi-NP were performed, using three concentrations (1.0, 3.0 and 5.0 mg/mL). The control used does not have nanoparticles. A notable bactericidal trend was observed, the number of colonies on the plate tended to decrease as the concentration increased.
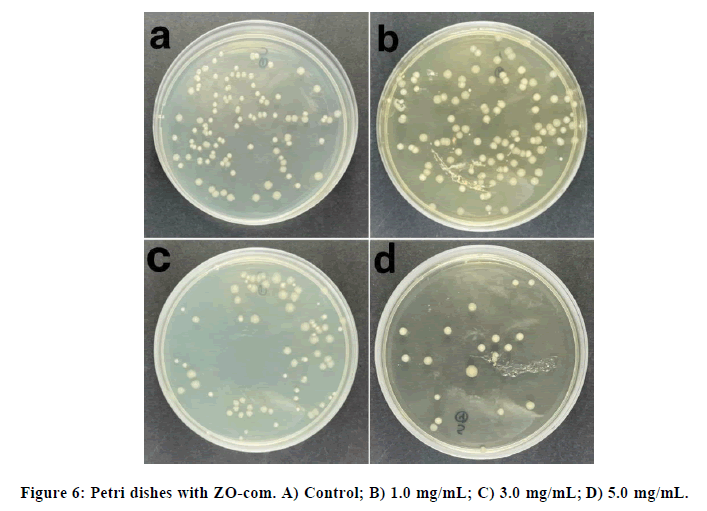
The liquid medium experiment with ZO-com was performed at three different concentrations, the control as the 100% of E.coli viability and the obtained plates are represented in Figure 6.
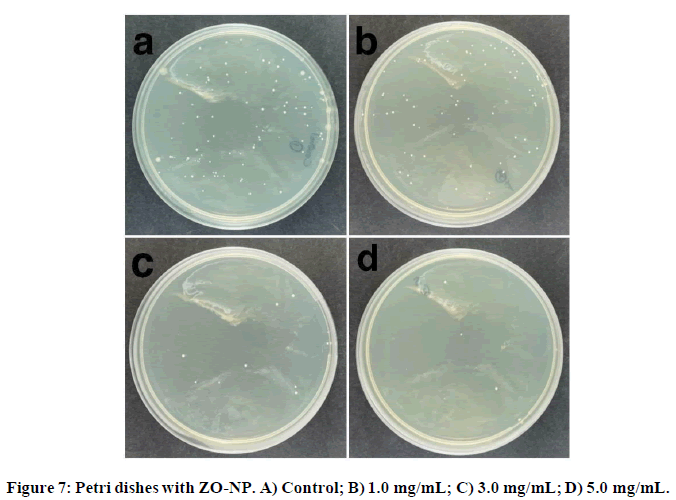
In Figure 6, a clear bactericidal tendency could be observed. The lowest E. coli viability is approximately at 40%. ZN- NP suspensions were added on the Petri dishes at three different concentrations and the results are shown Figure 7:
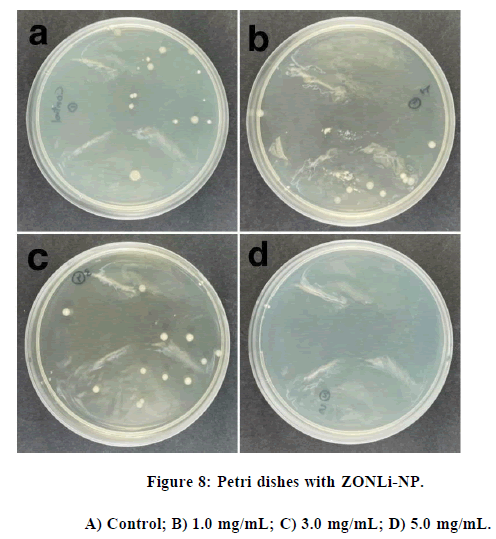
Similar to the results observed in ZO-com, an antibacterial trend is evident in Figure 7, but in this case, the results are even more promising. It is noteworthy that a concentration of 5 mg/mL, a 24% inhibition was noted, which nearly half the inhibition is observed with ZO-com. This phenomenon could be attributed to the smaller size of the synthesized nanoparticles compared to bulk ZnO, resulting in a higher specific surface area-to-volume ratio and, thus, enhanced physical interactions with the bacteria. Finally, the colony counting technique was applied to the codoped synthesized nanoparticles, and the results are displayed in Figure 8. The antibacterial trend observed in ZONLi-NP surpasses that of bulk ZnO.
The main objective of this method was to observe the bactericidal tendency, although a complete inhibition of bacterial growth was not observed in any of the studied concentrations, a clear antibacterial tendency of the nanoparticles was evident, especially at the highest concentration of NPs (5.0 mg/mL).
Summarizing, a common antibacterial trend was noticeable in this final technique in all three systems (Figure 9). As discussed, it was deduced that multiple mechanisms acted against bacteria. On the other hand, the disk diffusion method results in ZONLi-NP indicated that ROS diffusion played a predominant role in the antibacterial action. Considering the antibacterial trend in the colony forming unit technique, it was demonstrated that the ZnO systems also acted through physical principles when in direct contact with the bacteria.
As expected, the synthesized NPs were much more effective than the commercial. In the ZO-com at 5 mg/mL, the E.coli viability was approximately 40%, however, in the case of the ZO-NP and ZONLi-NP is approximately 25%, nearly half viability than the commercial one. With this, we could conclude that the synthesized nanoparticles were much more effective.
However, when comparing the two compositions of the synthesized nanoparticles in colony counting technique, unexpectedly there were not big differences in the antibacterial performance. Nevertheless, it was possible to observe some differences between them in the disk diffusion method. Codoped ZnO nanoparticles possess better characteristics thanks to their smaller size and bigger contact surface making easier the physical interaction between the ZnO systems and the bacteria. higher presence of ROS species and that is exactly that method showed.
Moreover, there are several mechanisms acting at different levels. In the disk diffusion method, the main difference is established by the diffusion of ROS in the codoped nanoparticles. In the colony counting experiment, the different tendencies are established by the size of the systems and their mechanical contact with bacteria.
Conclusion
The polyol method proved to be straightforward and efficient to nanoparticle synthesis. It was possible to observe the morphology, structure and properties of the systems by SEM and XRD techniques. In agreement with the literature, our results revealed several possible mechanisms in ZnO systems acting against the bacteria: Physical and chemical. The disk diffusion method, it was shown that the presence of ROS species stimulated the antibacterial tendency in the codoped nanoparticles. Also, in the colony counting technique, a clear trend of antibacterial behavior with an increase in the concentration of the nanoparticles is appreciated. On the one hand, when comparing the three ZnO systems, the main conclusion was that the action of the synthesized nanoparticles was much better than the bulk commercial ZnO. This could be tightly related with the much smaller size of the synthesized nanoparticles that could be controlled thanks to the synthesis method. The smaller size of the nanoparticles increases the surface of contact with the bacteria and therefore the physical interaction between the nanoparticicles and the bacteria is increased. Overall, these nanoparticles are promising materials with great potential for its use in coatings with antibacterial properties.
References
- Ventola CL. PT. 2015; 40(4): 27-283.
[Google Scholar] [PubMed]
- Laxminarayan R, Duse A, Wattal C, et al. Lancet Infect. Dis. 2013;13(12):1057-1098.
[Crossref] [Google Scholar] [PubMed]
- Xie Y, He Y, Irwin PL, et al. Appl. Environ. Microbiol. 2011;77(7);2325-2331.
[Crossref] [Google Scholar] [PubMed]
- Chieng BW, Loo YY. Mater. Lett. 2012;73(1):78-82.
- Wang ZL. Mater. Today. 2004;7(6):26-33.
- Krtschil A, Dadgar A, Oleynik N, et al. Appl. Phys. Lett. 2005;87(26). [Crossref]
- Radzimska AK, Jesionowski T. Materials. 2014;7(4):2833-2881.
[Crossref] [Google Scholar] [PubMed]
- Wiesmann N, Tremel W, Brieger J. J. Mater. Chem. B. 2020;8(23):4973-4989.
- Lawaetz P. Phys. Rev. B. 1972;5(10):4039-4045.
- Jara P, Jiménez RF, Ferreiro A, et al. Mater. Sci. Eng. B. 2024;299(1):116941.
- Mesaros A, Vasile BS, Talaman D, et al. Appl. Surf. Sci. 2019;471(1):960-972.
- Bhattacharya P, Neogi S. Rev. Chem. Eng. 2019;35(7):861-876.
- Li Y, Liao C, Tjong SC. Int. J. Mol. Sci.2020;21(22):8836.
[Crossref] [Google Scholar] [PubMed]
- Julca MA, Rivera I, Pérez OP, et al. MRS Proc. 2015;1784(1):mrss15-2136565.
- Cruz AO, Pérez OP. J. Electron. Mater. 2018;47(1):260-6265.
- Honglin L, Yingbo L, Jinzhu L, et al. J. Alloys Compd. 2014;617(1):102-107.
- Kumawat A, Misra KP, Chattopadhyay S. Mater. Technol. 2022;37(11): 595-1610.
- Lahmer MA. Appl. Surf. Sci. 2018;457(1):315-322.
- Yatskiv R, Grym J, Basinova N, et al. J. Lumin. 2023;253(1):119462.
- Ma H, Wallis HK, Diamond S, et al. Environ. Pollut. 2014;193(1):165-172.
[Crossref] [Google Scholar] [PubMed]
- Saqib NU, Adnan R, Shah I. Environ. Sci. Pollut. Res. 2016;23(16):15941-15951.
[Crossref] [Google Scholar] [PubMed]
- Sordello F, Berruti I, Gionco C, et al. Appl. Catal. B Environ. 2019;245(1):159-166.
- Genç H, Barutca B, Koparal AT, et al. Toxicol In Vitro. 2017;47(1):238-248.
[Crossref] [Google Scholar] [PubMed]
- Jin T, Sun D, Su JY, et al. J. Food Sci. 2009;74(1) M46-M52.
[Crossref] [Google Scholar] [PubMed]
- Xiao S, Liang T, Wong LS, et al. S. Afr. J. Chem. Eng. 2020;34(1): 6-71.
- Shaalan MI, Mahdy MME, Theiner S, et al. Acta Vet. Scand. 2017;59(1)49.
[Crossref] [Google Scholar] [PubMed]
- Hsueh YH, Ke WJ, Hsieh CT, et al. PLoS One. 2015;10(6):e0128457.
[Crossref] [Google Scholar] [PubMed]
- Syedahamed A, Karthikeyan C, Ahamed AP, et al. Nat. Publ. Gr. 2016;13(6):24312.
[Crossref] [Google Scholar] [PubMed]
- Jan T, Iqbal J, Ismail M, et al. Int. J. Nanomedicine. 2013;8(1):3679-3687.
[Crossref] [Google Scholar] [PubMed]
- Sirelkhatim A, Mahmud S, Seeni A, et al. Nano-Micro Letters. 2015;7(3):219-242.
[Crossref] [Google Scholar] [PubMed]
- Dong H, Chen YC, Feldmann C. Green Chem. 2015;17(8):4107-4132.
- Carrasco GF, Peña MR, Urbieta A, et al. Catalysts. 2021;11(1):71.
- Zhang L, Jiang Y, Ding Y, et al. J Nanopart Res. 2007;9(3):479-489.
- Vasile OR, Serdaru I, Andronescu E, et al. Comptes Rendus Chim. 2015;18(12):1335-1343.
- Applerot G, Lellouche J, Lipovsky A, et al. Small. 2012;8(21);3326-3337.
- Nosaka Y, Nosaka AY. Chem. Rev. 2017;117(17):11302-11336.
[Crossref] [Google Scholar] [PubMed]
- Kumar R, Umar A, Kumar G, et al. Ceram. Int. 2017;43(5):3940-3961.
- Li M, Zhu L, Lin D. Environ. Sci. Technol. 2011;45(5):1977-1983.
[Crossref] [Google Scholar] [PubMed]
- Ferreiro A, Flores-Carrasco G, Quevedo-López M, et al. Ceram. Int. 2023;49(21):33513-33524.
- El-Gohary FA, Abdel-Hafez LJ, Zakaria AI, et al. Infect Drug Resist. 2020;8(1):3485-3499.
- Taormina PJ, Niemira BA, Beuchat LR. Int. J. Food Microbiol. 2001;69(3):217-225.
- Samanta PK, Saha A, Kamilya T. Optik. 2015;126(18):1740-1743.
- Mclaren A, Valdes-Solis T, Li G, et al. J. Am. Chem. Soc. 2009;131(35):12540-12541.
- M. Hashim, C. Hu, X. Wang, et al. Appl. Surf. Sci. 2012;258(1):5858–5862.
- Raoufi D. Renewable Energy. 2013;50(1):932-937.
- Bachir S, Azuma K, Kossanyi J, et al. J. Lumin. 1997;75(1):35-49.
- Polarz S, Orlov A, Hoffmann A, et al. J. Mater. Chem. 2009;21(16):3889-3897.
- Khan U, Jan FA, Ullah R, et al. J Mater Sci Mater Electron. 2022;1(1):1-20
- Sharma A, Rai VN, Mani S, et al. Mater. Today: Proc. 2020;26(1):58-63.
- Sirajudeen J, Nagaswarupa HP, Kumar MA, et al. Mater. Today: Proc. 2017;4(11):11827-11836.
- Sahu J, Kumar S, Vats VS, et al. J. Lumin. 2022;243(1):118673.
- Ayana A, Gummagol NB, Patil PS, et al. J. Alloys Compd. 2022;922(1):166262.
- Baskaran C, Velu S, Kumaran K. Asian Pac. J. Trop. Dis. 2012;2:S658-S662.
- Azam A, Ahmed AS, Oves M, et al. Int J Nanomed. 2012;5(1):6003-6009.
- Jorgensen JH, Turnidge JD. J. Clin. Microbiol.2015;15(1):1253-1273.
- Ruangpan L, Tendencia E. SAFDC 2004.
- Sieuwerts S, De Bok FA. Lett. Appl. Microbiol. 2008;47(4):275-278.
- Zhang L, Jiang Y, Ding Y, et al. J Nanopart Res. 2010;12:1625-1636.