Original Articles: 2021 Vol: 13 Issue: 8
Development of ZnO-NiO Nanocomposites for the Electrochemical Detection of Dopamine using Modified Carbon Paste Electrode
Abstract
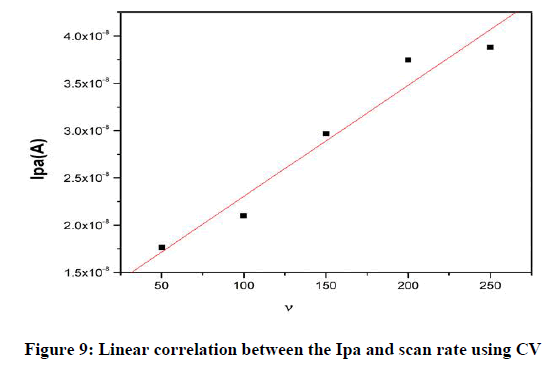
In this study, the ZnO-NiO nanocomposite modified carbon paste electrode was applied for the electrochemical determination of dopamine (DA) at pH 7.2 PBS solutions with scan rate 50 mVs-1. The voltammograms obtained during the oxidation studies revealed that ZnO-NiO NCs facile exhibits better catalytic function towards the oxidation of DA. The developed nanocomposite sensor offered high catalytic activity in sensing the dopamine MCPE application in the development of biosensors. The electrochemical responses of 5×10−5 M DA and its cyclic voltagramme was recorded in the potential range of -0.2 to 0.6 v vs. SCE in the 0.2 M phosphate buffer solution of pH 7.2 at the BCPE and the MCPE were measured at a scan rate of 50 mVs−1 by cyclic voltammeter (CV) technique was analyzed for BCPE and MCPE. The graph obtained exhibited good linearity between the scan rate (v) and the redox peak current for the ZnO-NiO nanocomposites MCPE with correlation coefficients of R=0.97811, which indicates that the electron transfer reaction was adsorption controlled process. It is expected that its good electrocatalytic behavior the ZnO-NiO NCs MCPE can be used in the development of biosensors and electroanalytical chemistry.
Keywords
Dopamine, Nanocomposites, Modified carbon paste electrode, Cyclic voltametry.
Introduction
There has been considerable interest in developing electrochemical techniques that have been proven to be significantly advantageous to biosensors and sensing the electrochemical detection of biomolecules [1-6]. Dopamine (DA) belongs to a member of the catecholamine family it is a neurotransmitter that plays an important role in the functions of the central nervous system and neurological disorders [7,8] that to in cardiovascular, memory and hormonal systems. The range of DA in human blood and serum is 10-6 -10-9 mol/L. The amount of dopamine decrease in the brains of patients causes Parkinson's disease and DA played an important role like neuro-transmission involves the conversion of an electrical impulse to a chemical event [9,10]. The level of DA in the sample is determined accurately with a simple, low cost with high selective and sensitivity developed sensors were required. In electrochemical techniques are more sensitive and effective towards the detection of the DA. During the detection of DA in extracellular fluids of the human body exhaust many interferences (ascorbic acid and uric acid). Due to the interferences modification on the surface of bare electrodes with novel electrocatalyst to increase the electrocatalytic activity towards the detection of the DA [11,12]. The electrocatalyst may an inorganic complexes, nanomaterials or composites, ionic liquids and conductive polymer materials. On modification of the surface of the bare electrode were carried out with carbon-based, metal and metal oxide nanoparticles with graphene-based biosensors are developed for the detection analysis of the DA [13-15]. Among different semiconductor metal oxide materials as the nanocomposite sensor offered high catalytic activity and blended oxide permit the possibility of tuning their materials properties according to the necessity for novel application [16-21]. Numerous mixed metal oxide nanoparticles, among that mixed ZnO and NiO nanocomposites (ZnO-NiO NCs) got extraordinary interests because of its lower expense, higher selectivity, high catalytic activity and the modification of an electrode with ZnO-NiO NCs can shift voltametric peaks in an analytically useful manner [22-29]. The developed mixed oxide ZnO-NiO NCs by precipitation method is used for MCPE to study voltametric detection of DA.
Experiment
All chemicals were obtained from Sigma Aldrich or Merck with high grade available and used without further purification. All solutions were prepared using deionized water. All experiments were carried out at room temperature.
Reagents and stock solutions
The reactant, triton X-100 [C14H22O(C2H4O)n], sodium hydroxide (NaOH), Dopamine hydrochloride, disodium hydrogen phosphate (Na2HPO4), sodium dihydrogen orthophosphate (NaH2PO4), silicone oil, graphite powder (mm particle size) were purchased from SD Fine Chemicals, Mumbai, India. The stock solution of dopamine (25 mM) was prepared in 0.1 M perchloric acid, phosphate buffer of pH 7.2 prepared in double-distilled water.
Preparation of ZnO-NiO nanocomposites
ZnO-NiO nanocomposites were prepared through the co-precipitation method. Experimental details followed as Zn (NO3)2.6H2O and Ni (NO3)2. 6H2O were used as the precursors and NaOH as the precipitant. Both the metal salts 0.1molL-1 in a molar ratio of 1:1 were dissolved in water, about 40molL-1 triton X-100 was added as a capping agent. The NaOH was added dropwise to the mixed solution at room temperature for 3 hours under constant stirring. The obtained slurry was centrifuged and the precipitate was washed. The obtained precipitate dried and then the powder is further heated in a silica crucible.
Instrumentation
A CHI 660D electrochemical work station (CH Instruments, Austin, USA) was used to take the measurements of cyclic voltammeter (CV), Electrochemical Impedance Spectroscopy (EIS) and Tafel. A conventional three-electrode system was employed, which consists of a modified CPE (ZnO-NiO nanocomposite carbon paste) as the working electrode; a saturated calomel electrode (SCE) as the reference electrode to measure cell potentials and a platinum wires an auxiliary electrode to measure current. XRD patterns were obtained on a Bruker D2 Phaser XRD system. SEM was studied using a scanning electron microscope (JEOL JSM 840).
Preparation of BCPE and MCPE (ZnO-NiO NCs)
The BCPE was prepared by uniform mixing of carbon powder with the proper amount of silicon oil in a mortar to develop a homogenous CPE. The carbon paste was packed into the cavity of the Teflon tube and then smoothed on a paper pad. The electrical contact was supplied by a wire of copper connected to the tube and the MCPE was prepared by adding 2,4,6,8 and 10 mg ZnO-NiO NCs to carbon powder and silicone oil with proper mixing of ratio mixture.
Results and Discussion
Characterization of ZnO-NiO NCs
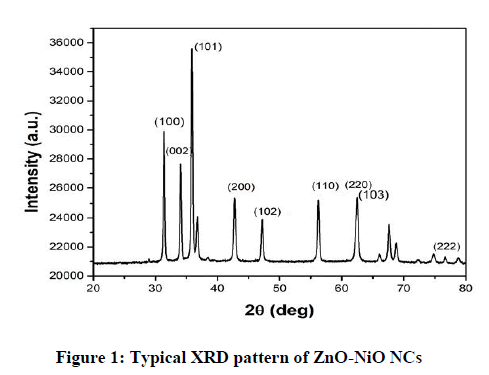
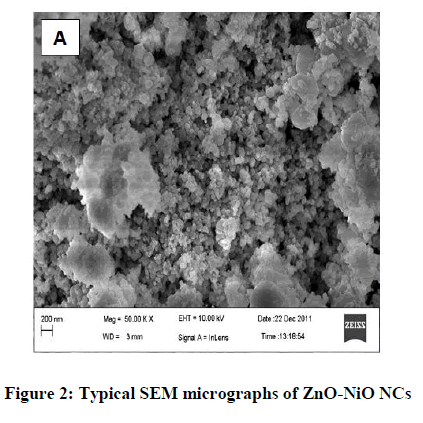
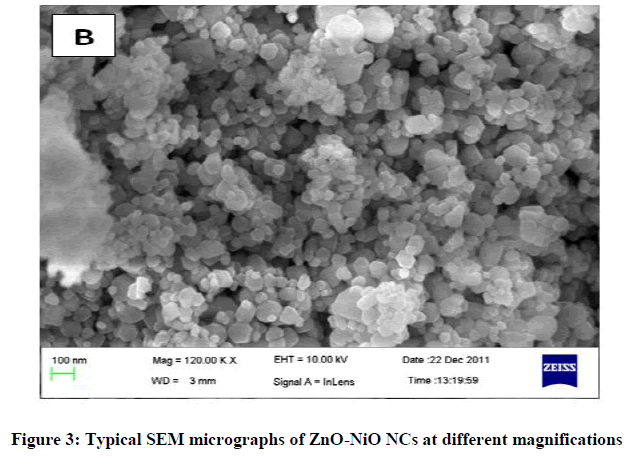
The XRD plot of the prepared ZnO-NiO NCs is shown in Figure 1. Some of the diffraction peaks like 31.7, 34.4, 36.2, 47.4 can be indexed to ZnO as per the JCPDS file 80-0075 but some other diffraction peaks like 43.2, 62.8, 75.1 are indexed to NiO as per the JCPDS file 78-0429. The dominance of ZnO over NiO is seen. XRD also shows that ZnO-NiO NCs are a mixture of two phases: a ZnO-based wurtzite phase and a NiO-based cubic phase with the rock salt structure this confirms the formation of ZnO-NiO NCs [30]. The sharp diffraction peak in the XRD pattern indicates the crystalline nature and the average crystallite size were found to be 14 nm. Figures 2 and 3 showed the SEM micrographs. In Figure 2 indicates the interconnected ultrafine particles, Figure 3 shows the cubical and spherical morphologies of ZnO-NiO NCs were observed. It demonstrates a nano-sized dimension with the agglomeration of composite particles.
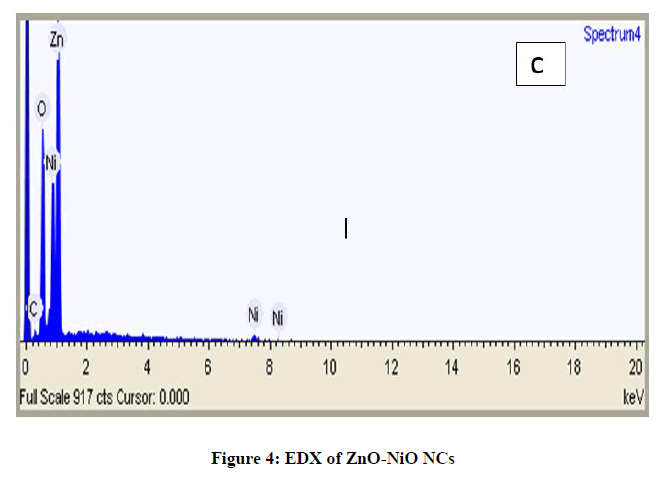
Figure 4 shows the EDX spectrum of ZnO-NiO NCs. The compound investigation of the arranged nanocomposites measured by EDX examination demonstrates that just Zn, Ni and oxygen signs have been distinguished, which demonstrated that the nanocomposites are for sure comprised of Zn, Ni and oxygen. No signal of secondary phase or polluting influence was recognized, subsequently proposed the high virtue of ZnO-NiO NCs and Figure 5 exhibits the Mass spectra of dopamine hydrochloride.
Effect of amount of ZnO-NiO NCs for investigation of DA
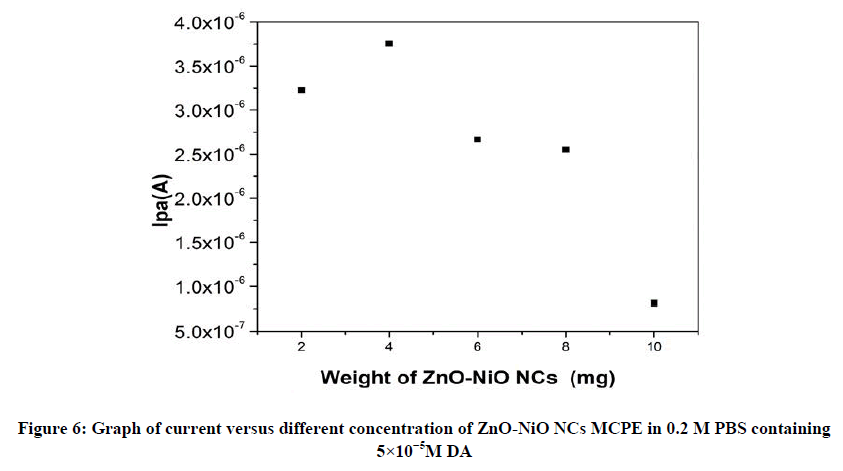
The effects of increasing amount of different mg concentration of ZnO-NiO NCs in a CPE were used CV studies of 5×10-5 M DA in a 0.2 M PBS at a scan rate of 50 mVs-1. The 4 mg of ZnO-NiO NC (MCPE) exhibit maximum current as compared with the 2,6,8 and 10 mg of ZnO-NiO NCs as shown in Figure 6 and this optimum response condition are maintained for CV studies during further investigation.
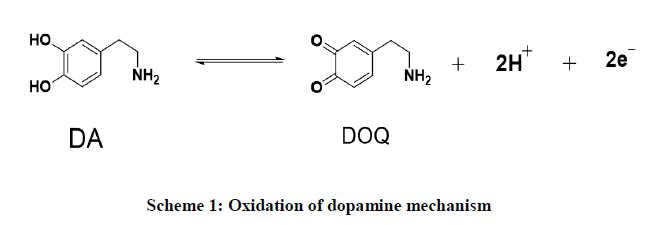
Electrochemical study of DA at BCPE and MCPE: The CV studies of 5×10−5 M DA in the potential range of -0.2 to 0.6 V in the 0.2 M PBS of pH 7.2 were measured at MCPE. The comparable peak potential differences ΔEp 0.1136 V for the MCPE are shown in Figure 7. At the BCPE the Epa 0.1046 V and the anodic peak currents significantly increased at the MCPE with the anodic peak potential 0.1100 V respectively. The result indicates MCPE exhibits good electrocatalytic activity than BCPE. Based on the literature results mentioned in Table 1 were compared with this study [31-34], it is clear the performance of ZnO-NiO NCs is better and can be effectively used as an alternative MCPE for the electrochemical sensor for the detection of dopamine. On oxidation of the DA undergoes to form dopaquinone through a transfer of electrons and holes during the CV analysis is shown in scheme.
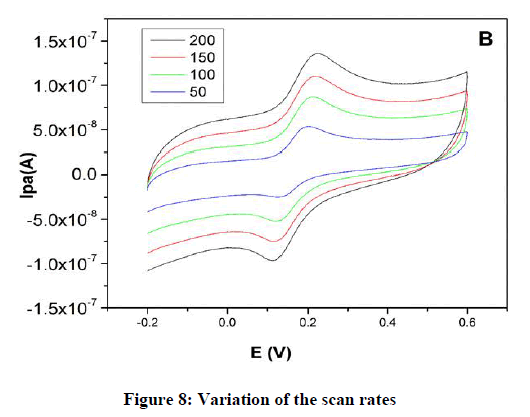
Effect of scan rate: The result of the scan rate influence on the CV studies for a peak current of DA in PBS at pH 7.2 at MCPE. Figure 8 shows an increase in the redox peak current Ipa 4.8 A at a scan rate of 0.005–0.250 Vs−1 for MCPE. The graph obtained exhibited good linearity between the scan rate and the redox peak current (Figure 8) for the MCPE with correlation coefficients of R2 0.978, which indicates that the electron transfer reaction was adsorption controlled process.
Determination of DA in DHI: In order to verify the analysis of DA in the pharmaceutical sample, the aimed MCPE was applied to determine DA in DHI. The CV studies of DHI solution in 0.2 M PBS of pH 7.2 was measured at MCPE at a scan rate of 50 mVs−1 by CV technique. CVs for BCPE in Figure 9 shows peak potential differences ΔEp 0.1457 V with Ipa 4.9 A compared to ZnO-NiO NCs MCPE, which shows peak potential differences ΔEp=0.0848 V with Ipa 5.3 A respectively. The results supported that the aimed method could be efficiently used for the detection of DA in commercial samples.
Interference study
The determination of several extraneous species as interfering compounds with the study of DA in the DHI solution was investigated and allowance limit is defined as the upper limit concentration of interfering species that cause an estimated relative error of ± 5% for the finding of DA. After the CV studies, we found no major disturbance for the finding of DA in the chosen compounds CaCl2 4000 μM, NaCl 4000 μM and KCl 5000 μM. Electrochemical response as the peaks remain unchanged after successive 20 cyclic voltammetric scans, confirms ZnO-NiO Ncs /MCPE has good stability (Figure 10).
Conclusion
In the present paper, synthesized the cubical and spherical structure through the co-precipitation process. Developed ZnO-NiO NCs were used in the modification of the electrode for the detection of DA. MCPE improved the sensitivity of the electrode and acting as a good electro-catalyst by sensing the sensing of the DA. MCPE worked as an electrochemical sensor for the detection of DA biomolecules. It is expected that it’s good electro-catalytic behavior for the application in the development of biosensors and electro-analytical chemistry. Due to the high stability and repeatability of the MCPE, it has the potential for the future development of nano-sensors for clinical research.
Acknowledgment
The authors wish to thank Dr. B.E. Kumaraswamy, Department of Industrial Chemistry, Kuvempu University, for his invaluable suggestions and moral support. The authors are also thankful to K.S. Institute of Technology, Bangalore for providing lab facility.
Conflict of Interest
Dr. Kiran Kumar S R, Centre for Nanosciences, Department of Chemistry, K.S. Institute of Technology, Bangalore, 560 062, India and authors declare that they have no conflict of interest.
References
- Hsu CY, Vasantha VS, Chen PY, et al. Bio Chem. 2009.
- Reddy S, Kumara Swamy BE, Jayadevappa H. Electrochim. 2012.
- Thomas T, Mascarenhas RJ, Nethravathi C, et al. J Electroanal Chem. 2011.
- Chand R, et al. Miner Eng. 2009.
- Motsi T, Rowson NA, Simmons MJH. Int J Miner Process. 2009.
- Chen CY, Chiang CL, Chen CR. Sep Purif Technol. 2007.
- Kapsimali M, Dumond H, Crom S, J socio bio. 2000.
- Volkow ND et al. Int J Eat Disord. 2003.
- Phillips PEM, Stuber GD, Helen MLAV, Wightman RM. J Nature. 2003.
- SellbachAN, Boyle RS, Silburn PA, J phar col. 2006.
- Xu TQ, et al. Electrochim Act. 2014.
- Yang L, Liu D, Huang. J Bio Chem. 2014.
- Oztekin Y, et al. Electrochim Act. 2012.
- Xin Y, Li Z, Wu W, et al. Biosens Bioelectron. 2017.
- Wang Y, Zhang Y, Hou Cet al. RSC Adv. 2015.
- Tasdelen C, S. Aktas S, Acma E, et al. J Hydrometallurgy. 2009.
- Ai L, Zeng Y. Chem Eng J. 2013.
- Karimi-Maleh H, Biparva P, Hatami M. Biosens Bioelectron. 2013; 48, 270-275.
- Shalygina, Nazarov IV, Baranov AV, et al. J Sol-Gel Sci Technol. 2017; 81(2), 333-337.
- Ohta H, et al. App Phys Lett. 2003; 83(5), 1029-1031.
- Wang JY, et al. App Phys Lett. 2009.
- Gupta RK, Ghosh K, Kahol PK. Phys E Syst. 2008; 41(4), 617-620.
- Zhang Z, et al. J Mater Chem. 2012.
- Wang H, et al. Chem Soc Rev. 2019.
- Ulubay S, Dursun Z. J Mater Chem. 2010.
- Li M, et al. Nanoscale. 2011; 3(10), 4240-4246.
- Frackowiak E. Chem Phys. 2007.
- Hu CC, Chen WC, Chang KH. J Electrochem Soc. 2004; 151(2), 281.
- Nam KW, Kim KH, Lee ES, et al. J Power Sources. 2008.
- Kumar KY, Muralidhara HB, Nayaka YA. J Power Sources. 2013.
- Kiran Kumar SR, Prashanth MK, Muralidhara HB, et al. Surf Eng Appl Electrochem. 2016.
- Ranganatha S, Kumar K, Mamatha GP. J Power Sources. 2015; 7(2), 175-185.
- Kumar RK, Kumar KY, Anantha S, et al. J Chem Bio Phy Sci. 2016; 6(3), 821-833.
- Kiran Kumar SR, et al. J Sci Adv Mater Devices, 2017.