Original Articles: 2022 Vol: 14 Issue: 3
Development of UV Visible Spectrophotometric Method for Estimation of Cefipime Hydrochloride in Bulk and Dosage Form
1Department of Pharmacy, Sree Chaithanya Institute of Pharmaceutical Sciences, Telangana, Indias
- Corresponding Author:
- Samatha K
Department of Pharmacy, Sree Chaithanya Institute of Pharmaceutical Sciences, Telangana, Indias
Received: 22-Mar-2022, Manuscript No. JOCPR-22-s52726; Editosr assigned: 24-Mar-2022, PreQC No. JOCPR-22-52726 (PQ); Reviewed: 05-Apr-2022, QC No. JOCPR-22-52726; Revised: 11-Apr-2022, Manuscriptss No. JOCPR-22-52726 (R); Published: 18-Apr-2022, DOI: 10.37532/0975-7384.2022.14(3).019.
Abstract
The three simple, accurate, rapid, and sensitive UV-Visible spectrophotometric methods have been developed for estimation of Cefepime Hydrochloride in bulk and pharmaceutical formulation. It describes development of 3 new visible spectrophotometric based on different chemical reactions namely cefepime hydrochloride and reagent (1,10 Phenanthroline α-napthylamine (BM reagent). Among 3 methods CFP1 (512 nm) and CFP2 (565 nm) more sensitive followed by CFP3 (510 nm) because of higher λmax compared to methodsCFP1 and CFP2. The proposed 3 methods are based on exploitation of 3 structural feature of molecule (Aromatic amino group and oxidized nature of CFP in developing simple and sensitive method for assay of Cefepime hydrochloride. The result of Analysis of pharmaceutical formulation reveals that the proposed methods are suitable for Analysis. All these methods were used to produce coloured species. The methods were extended for determination of Cefepime hydrochloride in parenteral preparation was chosen. All the proposed methods are simple and reliable and provide a wide choice for determination of Cefepime hydrochloride in bulk or in pharmaceutical formulation depending on specific analytical situation
Keywords
Cefepime hydrochloride; UV-Visible spectrophotometer; BM reagent; α-napthyamine; λmax
Introduction
Cefepime is a fourth-generation cephalosporin antibiotic developed in 1994. Cefepime is active against Grampositive and Gram-negative bacteria, with greater activity against both than third-generation antibiotics. Cefepime is usually reserved to treat severe nosocomial pneumonia, infections caused by multi-resistant microorganisms (e.g. Pseudomonas aeruginosa) and empirical treatment of febrile neutropenia [1].
Drug Profile
Cefipime Hydrochloride
Category: Fourth generation of cephalosporins
s C19H28Cl2N6O6S2
Molecular structure:

Chemical name: (6R-(6α,7β(Z))-1-((7-(((2-amino-4-thiazolyl)(methoxyimino)acetyl)amino)-2-carboxy-8-oxo-5- thia-1-azabicyclo(4.2.0)oct-2-en-3-yl)methyl)-1-methylpyrrolidinium chloride monohydrochloride.
MATERIALS AND METHODS
Materials Required
Pure sample: Cefepime Hydrochloride.
Market sample: Maxipime 1 g vial (smith Kline beecham), batch no g42343 labelled to contain Cefepime Hydrochloride equal to 1 g of cefepime.
Apparatus: Electronic analytical balance, distilled water, pipette, volumetric flask, beaker, measuring cylinder
Instrumentation: Shimadzu UV Visible Spectro Photometer (UV 1800) with 1 cm matched quartz cells were used for special measurement. The spectrophotometer was equipped with UV probe software.
Chemicals and reagents: Methanol, sodium hydroxide. Ethanol, sodium nitrite, Hydrochloric acid, ammonium sulphate, ferric chloride hexa hydrate,1,10 phenanthroline,α-napthylamine,BM reagent(n-1-napthyl ethylene diamine dihydrochloride).
Solvent system: 0.1 N Methanol, 1 ml HCl, 1 ml sodium nitrite.
Preparation of Standard Drug Solutions
For methods CFP1 and CFP2: A standard stock solution of 1 mg/ml was prepared by dissolving 100 mg of cefipime hydrochloride in 100 ml of distilled water and this stock solution was diluted stepwise with distilled water to obtain the working standard solutions of concentration 100 mcg/ml CFP1 and CFP3 and 50 mcg/ml for CFP2.
Preparation of working standard solution: Accurately measured 1 ml of standard stock solution was pipette out into 10 ml volumetric flask and diluted using 0.1 N Methanol up to the mark to prepare the concentration of 100 mcg/ml CFP1 and CFP3 and 50 mcg/ml for CFP2.
Preparation of reagents: All the chemicals and reagents used were of analytical grade and the solutions were prepared in double distilled water [2] (Tables 1-3).
| Sodium nitrite (S.d fine chem.,0.2%w/v.2.89 × 10-2 M) |
Prepared by dissolving 200 mg of sodium nitrite 100 ml of distilled water. |
| HCL (S.d,fine-chem,1M) | Prepared by dissolving 3.65 ml of hydrochloric acid in 100 ml of distilled water. |
| Ammonium sulphate (S .d .fine-chem,0.5%w/v4.38 × 10-2 M) |
Prepared by dissolving 500 mg of ammonium sulphate in 100 ml distilled water. |
| Α-napthyl amine(α-NA) (S .d. fine-chem.0.2%1.39 × 10-2 M) |
Prepared by dissolving 200 mg of α-napthylamine in 100 ml methanol. |
| Sodium nitrite (S.d fine chem.,0.2%w/v.2.89 × 10-2 M) |
Prepared by dissolving 200 mg of sodium nitrite 100 ml of distilled water. |
| HCL (S.d,fine-chem,1 M) |
Prepared by dissolving 3.65 ml of hydrochloric acid in 100 ml of distilled water. |
| Ammonium sulphate (S.d.fine-chem,0.5%w/v4.38 × 10-2 M) |
Prepared by dissolving 500 mg of ammonium sulphate in 100 ml distilled water. |
| BM Reagent (S.d. fine-chem.0.1%w/v,3.85 × 10-3 M) |
Prepared by dissolving 100 mg of BM Reagent in 100 ml of distilled water. |
| Ferric chloride hexa-hydrate (s.d.finechem.,0.03 M) | Prepared by dissolving 80 mg of ferric chloride in 100 ml distilled water. |
| 1,10-phenanthroline (s .d. finr-chrm.,0.1 M) | Prepared by dissolving 1.98 gm of 1,10 Phenanthroline in slightly warm distilled water to facilitate proper mixing. |
Recommended procedure: After systematic and detailed study of the various parameters involved, as described under results and discussions, the following procedures were recommended for the assay of CFP in bulk samples and pharmaceutical formulations.
For Bulk Samples
Aliquots of standard drug solution of cefipime hydrochloride (0.5-4.0 ml) (100 mcg/ml) were taken and transferred into series of graduated test tubes. To each test tube 1 ml (1 M) of hydrochloric acid and 1 ml sodium nitrite (0.2% w/v) were added, mixed and cooled in ice-bath for 5 mins, then 1 ml of ammonium sulphamate (0.5% w/v) was added and the test tubes were kept at room temperature for 3 mins for complete neutralization of the excess nitrous acid formed in the reaction [3-5]. Then finally 1 ml of α-naphthylamine solution (0.1% w/v) was added and mixed well, immediately pink color was developed. The volumes in each test tube were adjusted to 10 ml with distilled water. The absorbances of the solutions were measured at 512 nm against reagent blank, and the calibration curve was plotted. Similarly the absorbance of the sample solution was measured, and the amount of cefipime hydrochloride was determined by referring to the calibration curve [6].
Method B: Aliquots of standard drug solution of cefipime hydrochloride 0.5-4.0 ml (50 mcg/ml) were taken and transferred into series of graduated test tubes. To each test tube 1 ml of hydrochloric acid (1 M) and 1 ml sodium nitrite (0.2% w/v) were added, mixed and cooled in ice-bath for 5 mins, then 1 ml of ammonium sulphamate (0.5% w/v) was added and the test tubes were kept at room temperature for 3 mins for complete neutralization of the excess nitrous acid formed in the reaction [7]. Then finally 2 ml of BM reagent solution (0.1% w/v) was added and mixed well. The volumes in each test tube were adjusted to 10 ml with distilled water. The absorbances of the purple colored solutions were measured at 565 nm against reagent blank, within 30 mins and the calibration curve was plotted. Similarly thse absorbance of the sample solution was measured, and the amount of cefipime hydrochloride was determined by referring to the calibration curve [8].
Method C: Aliquots of standard drug solution of cefipime hydrochloride 0.25-4.0 ml (100 mcg/ml) were taken and transferred into series of graduated test tubes. To each test tube 2 ml of Ferric chloride (0.03 M) and 2 ml of 1, 10- Phenanthroline (0.1 M) was added. The test tubes were allowed to stand in water bath at 600ºC for 15 mins. The test tubes were then cooled to room temperature and the solutions were made up to 10 ml with distilled water. The absorbance of the red colored chromogen was measured at 510 nm against reagent blank and a calibration curve was constructed. The absorbance of the sample solution was measured, and the amount of cefipime hydrochloride was determined by referring to the calibration curve [9].
For pharmaceutical formulations: Powder for Injection containing cefipime hydrochloride was successfully analyzed by the proposed methods.
Method CFP1, CFP2 and CFP3: The methods were extended for the determination of cefipime hydrochloride in parenteral preparation (Cefrom 1.0, Aventis) was chosen. The powder for injection contains sodium carbonate as excipients. A quantity of the powder equivalent to 100 mg was accurately weighed and transferred to 100 ml volumetric flask and made up to mark with distilled water. The contents of the flask were thoroughly mixed and filtered. Appropriate volume of the filtrate was suitably diluted to get a 100 mcg/ml concentration [10-12]. The sample solution was analyzed as described, in the above mentioned methods (Table 4).
| Pharmaceutical formulation | Reference method |
|---|---|
| Powder for injection | Cefipime vial (Cefrom 1.0, Aventis) containing 1 gm of drug. Powder equivalent to 50 mg of drug was accurately weighed and taken in a 50 ml volumetric flask. Added 1 ml of 1.0 N NaOH to dissolve and then Volume was made up to 50 ml with distilled water to get concentration of 1 mg per ml and filtered. From this stock solution, serial dilution was made in the concentration range of 20 to 100 mcg/ml and their absorbances were measured at 230 nm. The concentration of the drug was computed from its calibration graph. |
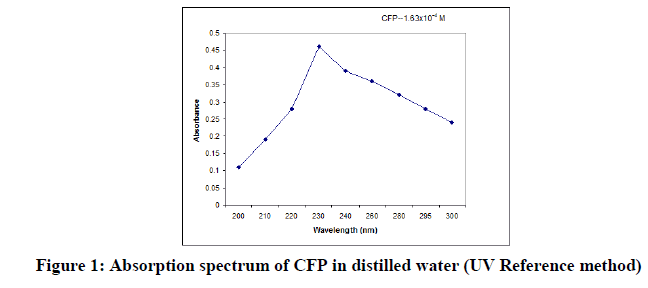
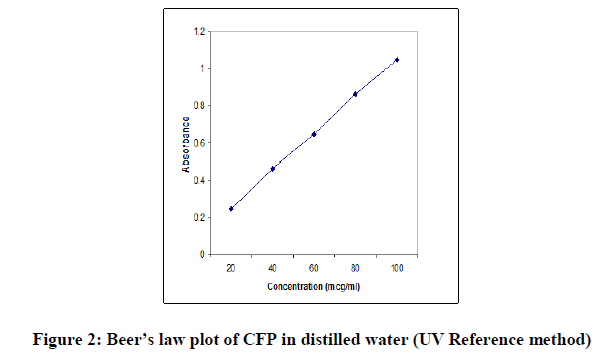
The concentration of drug was computed from its calibration curve. The absorption spectrum and the Beer’s law plot of the bulk drug pertaining to the reference method are presented in Figures 1 and 2.
RESULTS AND DISCUSSION
Spectral Characteristics
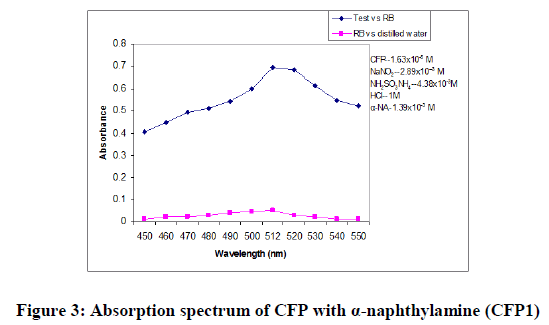
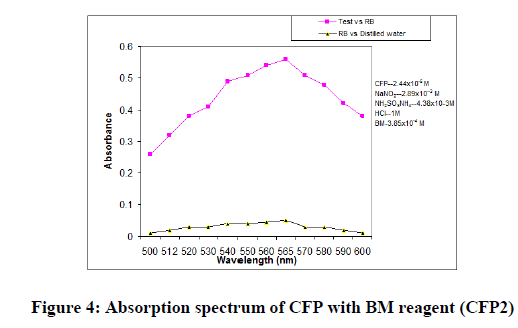
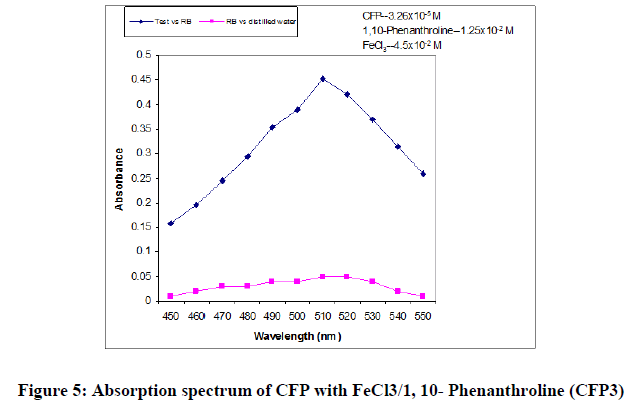
In order to ascertain the optimum wavelength of maximum absorption (λmax) of the colored species formed in each one of the four methods, specified amounts of CFP in final solution (100 mcg/ml, 150 mcg/ml and 200 mcg/ml for methods CFP1, CFP2 and CFP3 respectively) were taken and the colors were developed separately following the above mentioned procedures [13]. The absorption sspectra were scanned on a spectrophotometer in a wavelength region of 360-800 nm against a corresponding reagent blank. The reagent blank absorption spectrum of each method was also recorded against appropriate solvent. The results were graphically presented in Figure 3,4 and 5.
Parameters fixation: In developing these methods, a systematic study of the effects of various parameters in the methods concerned were undertaken by varying one parameter at a time and controlling all other parameters to get maximum color development, minimum blank color, reproducibility and reasonable period of stability of final colored species formed. The following studies were conducted [14].
For CFP1: The optimum conditions in these methods were fixed based on the study of the effects of various parameter such as volume of α-naphthylamine, volume of sodium nitrite, time required for maximum color requirement, order of addition of reagents and the stability of the colored species after final dilution were studied and the results are incorporated in Table 5.
| Parameter | Optimum range | Conditions in procedure | Remarks |
|---|---|---|---|
| λmax (nm) | 512 nm | 512 nm | -- |
| Volume of 1.39 × 10-4 M α-naphthylamine required for color development | 0.5-3.0 ml | 1 ml | More than 1.0 ml results in high blank values. |
| Volume of 2.89× 10-3 M Sodium nitrite solution, needed for diazotization |
0.5-2.0 ml | 1.0 ml | The absorbance increases steadily over the range of 0.5 to 1 ml of NaNO2 solution and then remains constant even after adding more. |
| Volume of 1 M HCl, required for diazotization. | 0.5-2.0 ml | 1 ml | Minimum amount of 1 ml of HCl is necessary to maintain acidic conditions (when no acid is present, diazotization does not occur). |
| Volume of 4.38 × 10-3 M Ammonium sulphamate | 0.5-2.0 ml | 1 ml | 1 ml of ammonium sulphamate is sufficient to neutralize the excess nitrous acid. |
| Time required for diazotization | 2-25 min | 5 min | The same absorbance is noticed over the time interval 2-15 min. For 15-30 min the results are erratic. On standing overnight the color disappears indicating the benzene diazonium chloride analogue had undergone decomposition. |
| Effect of temp. on diazotization | 5-20ºC | 5-10 ºC | The absorbance increases when kept at temp. of 5-10ºC, so it is necessary to cool the solution in ice. Low absorbance values are obtained when the above temp. is not maintained. |
| Solvent for final dilution | Distilled water | Distilled water | |
| Stability of the colored species after final dilution | 10-60 min | 40 min | After 40 min, the absorbance starts to decrease. |
For CFP2: The optimum conditions in these methods were fixed based on the study of the effects of various parameter such as volume of BM reagent, volume of sodium nitrite, time required for maximum color requirement [15,16], order of addition of reagents and the stability of the colored species after final dilution were studied and the results are incorporated in Table 6.
| Parameter | Optimum range | Conditions in procedure | Remarks |
|---|---|---|---|
| λ max (nm) | 565 nm | 565 nm | -- |
| Volume of 3.85 × 10-4 M BM reagent required for color development |
0.5-3.0 ml | 2 ml | More than 2.0 ml results in high blank values (color becomes more intense in blank). |
| Volume of 2.89 × 10-3 M Sodium nitrite solution. |
0.5-2.0 ml | 1.0 ml | The absorbance increases steadily over the range of 0.5 to 1 ml of NaNO2 solution and then remains constant even after adding more. |
| Volume of 1 M HCl | 0.5-2.0 ml | 1 ml | Minimum amount of 1 ml of HCl is necessary to maintain acidic conditions (when no acid is present, diazotization does not occur). |
| Volume of 4.38 × 10-3 M Ammonium sulphamate | 0.5-2.0 ml | 1 ml | 1 ml of ammonium sulphamate is sufficient to neutralize the excess nitrous acid. |
| Time required for diazotisation | 2-25 min | 5 min | The same absorbance is noticed over the time interval 2-15 min. For 15-30 min the results are erratic. On standing overnight the color disappears indicating the benzene diazonium chloride analogue had undergone decomposition. |
| Effect of temp. on diazotization | 5-20°C | 5010°C | The absorbance increases when kept at temp of 5-10°C, so it is necessary to cool the solution in ice. Low absorbance values are obtained when the above temp is not maintained. |
| Solvent for final dilution | Distilled water | Distilled water | |
| Stability of the colored species after final dilution | 5-30 min | 30 min | After 30 min, the absorbance starts to decrease. |
For CFP3: The optimum conditions were fixed based on the study of effects of various parameters such as volume and concentration of 1,10-phenathroline, heating time and temperature for color development and stability of the colored species after final dilution, by measuring the absorbances at 510 nm against reagent blank and results were incorporated in Table 7.
| Parameter | Optimum range | Conditions in procedure | Remarks |
|---|---|---|---|
| λmax (nm) | 510 nm | 510 nm | -- |
| Volume of 0.03 M Ferric Chloride required for maximum color development | 0.5 ml-3.0 ml | 2.0 ml | <3.0 ml of Ferric Chloride results in decrease of absorbance. |
| Effect of Volume of 0.02 M 1,10-phenanthroline required for complex formation | 0.5-3.0 ml | 2.0 ml | 1.5 ml of 1,10-phenathroline was found to be necessary for maximum absorbance and to cover broad range of Beer’s law limit. No added advantage was observed with more than 1.5 ml |
| Effect of temperature and time | 60-80°C 10-20 min | 60°C 15 min | No color development was found at room temperature. Heating in boiling water bath for 15 min was required to obtain maximum color development and better results (sensitivity and reproducibility). |
| Order of addition of reagents | Drug, FeCl3 and 1,10-phenanthroline | Drug, FeCl3 and 1,10-phenanthroline | Change in order of addition has less effect. |
| Stability of colored species, after final dilution | 45 min- 8 hrs | 2 hrs | The colored complex was stable for a period of 2 hrs, the absorbance decreased slowly with time after 2 hrs. |
Optical Characteristics
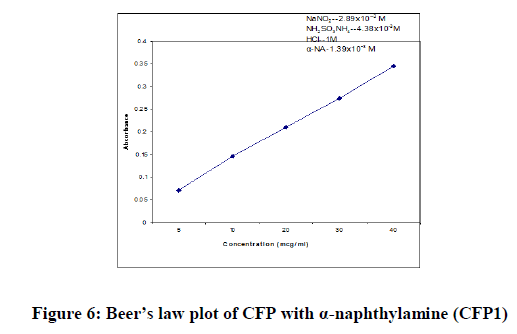
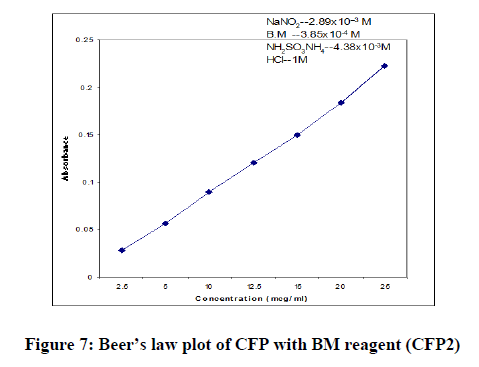
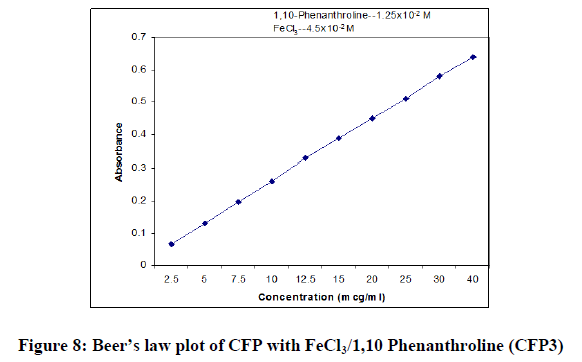
In order to test whether the colored species formed in the proposed methods adhere to Beer’s law, the absorbance at appropriate wavelength of a set of solutions containing varying amounts of CFP and specified amounts of reagents (as given in the recommended procedures for each method) were recorded against the corresponding reagent blanks. The Beer’s law plots of these systems were recorded graphically.
Precision: The precision of the proposed methods was ascertained from the absorbance values obtained by actual determination of six replicates of a fixed amount of CFP in final solutions (100 mcg/ml, 50 mcg/ml and 100 mcg/ml for CFP1, CFP2 and CFP3 respectively). The percent relative standard deviation and percent range of error (0.05 and 0.01 confidence limits) were calculated for the proposed methods and incorporated in the Table 5.
Accuracy: To determine the accuracy of the proposed methods, different amounts of bulk samples of CFP within the Beer’s law limits were taken and analyzed by the proposed methods. The results (percent error) are recorded in Table 8.
| Parameters | CFP1 | CFP2 | CFP3 |
|---|---|---|---|
| lmax (nm) | 512 | 565 | 510 |
| Beer’s law limits (mcg/ml) | 05-40 | 2.5-20 | 2.5-40 |
| Molar absorptivity (l/mol.cm) | 7.52 × 103 | 4.69 × 103 | 1.26 × 103 |
| Sandell’s sensitivity (micrograms/cm2/0.001 absorbance unit) |
0.6849 | 0.8928 | 0.4065 |
| Optimum photometric range (mcg/ml) | 06-38 | 18-04 | 16-04 |
| Regression Equation* (Y) Slope (m) |
7.4 × 10-3 | 8.71 × 10-3 | 1.54 × 10-2 |
| Standard deviation on slope (Sm) | 4.71 × 10-4 | 1.96 × 10-5 | 1.66 × 10-4 |
| Intercept (c) | 5.37×10-2 | 9.71 × 10-3 | 9.43 × 10-2 |
| Standard deviation on intercept (Sc) | 1.51 × 10-2 | 2.99 × 10-3 | 2.83 × 10-2 |
| Correlation Coefficient (r) | 0.9998 | 0.9989 | 0.9999 |
| Precision (%Relative Standard Deviation) | 0.14 | 0.774 | 0.282 |
| Standard error of mean | 0.0185 | 0.0043 | 0.017 |
| % range of error (confidence limits)* 0.05 level; 0.01 level | 0.972 1.524 |
0.313 0.492 |
0.246 0.386 |
| % error in bulk samples | 0.71 | -0.14 | -0.18 |
Note: *Y=mx+c, where X is the concentration in micrograms/ml and Y is absorbance unit.
Interference studies: The effect of a wide range of concomitants and additives usually present in the formulations for the assay of CFP under optimum conditions were investigated separately. In all the methods prepared, the commonly used concomitants and additives in the preparation of formulations did not interfere with the assay of CFP by proposed methods.
Analysis of formulations: Commercial formulations (Film coated Tablet, ZERODAX®, 200 mg), containing CFP were successfully analyzed by the proposed methods. The results obtained by the proposed and reference methods for formulations were compared statistically with t- and F- tests and found not to differ significantly. The results were summarized in Table 9.
| Labelled Amount | % found by the proposed methods* | % found by the reference method | % Recovery ± RSD** Proposed method | % recovery found by the reference method | |||||
|---|---|---|---|---|---|---|---|---|---|
| CFP 1 | CFP 2 | CFP 3 | CFP 1 | CFP 2 | CFP 3 | ||||
| ZEDORAX® | 200 mg | 100.19 ± 0.23 | 100.26 ± 0.38 | 100.52 ± 0.27 | 100.23 ± 0.36 | 99.86 ± 0.43 | 99.71 ± 0.47 | 100.49 ± 0.51 | 100.69 ± 0.33 |
| TABLET | T=0.25 | t=0.10 | t=1.88 | - | - | - | - | - | |
| F=2.44 | F=1.11 | F=1.77 | - | - | - | - | - | ||
| 200 mg | 99.99 ± 0.26 | 100.1 ± 0.17 | 100.79 ± 0.63 | 100.21 ± 0.38 | 99.96 ± 0.17 | 99.59 ± 0.47 | 100.43± 0.31 | 100.41 ± 0.43 | |
| t=1.15 | t=0.64 | t=1.92 | - | - | - | - | - | ||
| F=2.13 | F=4.99 | F=2.74 | - | - | - | - | - | ||
| 200 mg | 100.44 ± 0.45 | 100.33 ± 0.30 | 100.35 ± 0.44 | 100.3 ± 0.33 | 100.12 ± 0.13 | 99.75 ± 0.59 | 100.04 ± 0.59 | 100.49 ± 0.38 | |
| t=0.61 | t=0.20 | t=1.2 | - | - | - | - | - | ||
| F=1.85 | F=1.21 | F=1.21 | - | - | - | - | - | ||
Note: **Average±standard deviation of six determinations, the t- and F-test values refer to comparision of the proposed method with the reference method. Theoretical values at 95% confidence limit, t=2.57, F= 5.05
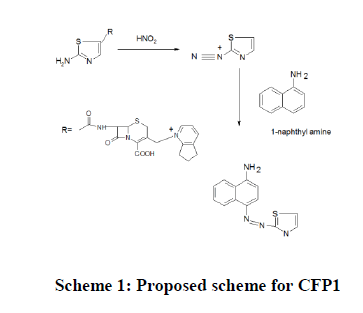
Chemistry of the colored species CFP1: Primary aromatic amines133 ArNH2, undergo reaction with an acidic nitrous acid solution to give diazonium salt. The highly reactive diazonium salt Ar-NH2+Cl- will react with a second organic compound, α-NA containing a carbon atom of high electron density to yield a colored diazo compound. This reaction is called diazo-coupling reaction. CFP which contains a primary aromatic amino group undergoes diazotization in the presence of nitrous acid and hydrochloric acid to yield a diazonium intermediate, which then couples with α-NA, to yield a colored chromogen (Scheme 1).
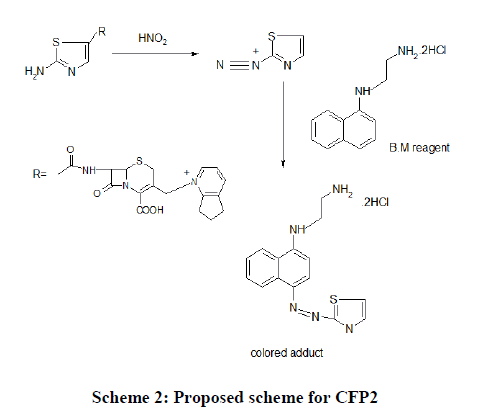
CFP2: The technique involves the diazo coupling of the amino group present in CFP, in acidic nitrous acid solution to yield a diazonium salt, which undergoes electrophilic substitution to a high electron density carbon compound namely B.M reagent (N-(1-naphthyl) ethylenediamine) to yield a colored chromogen. The excess nitrous acid is destroyed by adding ammonium sulphamate (Scheme 2).
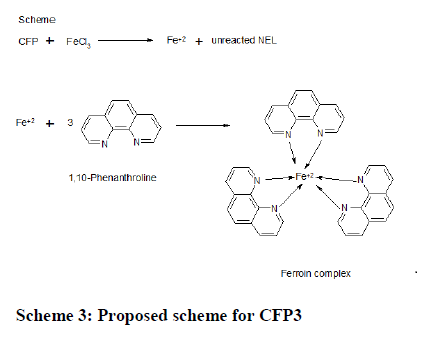
CFP3: Step I: When treated with known excess of Fe(III) drug undergoes oxidation giving oxidation products of drug (CFP) inclusive of reduced form of Fe(III) i.e Fe(II). Ods are suitable for their analysis with virtually no interference of the usual additives, presented in pharmaceutical formubesides unreacted Fe(III). Fe(II) has a tendency to give colored complex on treatment with 1,10-phenanthroline.
CFP + Fe(III) Oxidation product of CFP + Fe(II)+Unreacted Fe(III)
Oxidation product of CFP + Fe(II)+Unreacted Fe(III)
Step II: The next step concerns with the estimation of Fe (II) with 1, 10-phenanthroline which forms colored tries complex (Ferroin)(Scheme 3).
CONCLUSION
There are only few methods reported for the assay of CFP utilizing visible Spectrophotometry and hence there is dearth of analytical methods for the determination of CFP. It can be seen from the results presented all the proposed methods have good sensitivity, precision and accuracy. Results of the analysis of pharmaceutical formulations reveal that the proposed methylations.
Among the three proposed methods, CFP1 and CFP2 are the most sensitive followed by CFP3. Methods CFP2 has the advantage of higher λmax compared to methods CFP1 and CFP3. As the three proposed methods are based upon the exploitation of different structural features of the molecule, each method has its own merits in different situations. All the proposed methods are simple, sensitive and reliable and provide a wide choice for the determination of CFP in bulk or in pharmaceutical formulations, depending on the needs of the specific analytical situation.
REFERENCES
- Rabouan-Guyon RSM, Guet AF, Courtois PY, et al. Int J Pharm. 1997;154:185-190.
[Cross Ref][Google Scholar]ss
- Yahav D, Paul M, Fraser A, et al. Lancet Infect Dis. 2007;7:338-348.
- Evagelou V, Tsantili -Kakoulidou A and Koupparis M. J Pharm Biomed Anal. 2003;31:1119-1128.
- Sprauten PF, Beringer PM, Louie SG, et al. Antimicrob Agents Chemother. 2003;47:1991-1994.
- Sunnyvale CA. J chem. 2005.
- Palacios JJ, Sanchez MC and Carranza JH. Electro analysis. 2000;12:296-300.
- Tseng SH, Yang YH, Chen YR, et al. Electrophoresis. 2004;25:1641-1647.
- Yang YH, Wu WY, Yeh HH, et al. Electrophoresis. 28:1788-1797.
- Linger EL, Monteil H and Jehl F. J Chromatogr. 1997;690:181-188.
- Calahorra B, Campanero MA, Sadaba B, et al. Biomed Chromatogr. 1999;13:272-275.
- Chang L, Chou MH, Lin MF, et al. J Chromatogr. 2001;914:77-82.
- Breilh D, Lavallee C, Fratta A, et al. J Chromatogr. 1999;734:121-127.
- Valassis IN, Parissi-Poulou M and Macheras P. J Chromatogr B Biomed Sci Appl. 1999;72(1):249-255.
- Kinowski CJM, Lefrant JY and Bressolle F. J Chromatogr B Biomed Sci Appl. 2000:377-386.
- Gonzalez AO, Palacios FJJ, Mochon MC, et al. J Pharm Bio Anal. 2004;36(1):117-123.
- Heyman and Liu L. J Chrom B. 2010; 878:1623- 1628.