Original Articles: 2022 Vol: 14 Issue: 10
Comparative analysis and impact assessment on the electricity price of traditional carbon capture methods and that of direct precipitation of CO2 with the addition of Ca(OH)2 in the scope of a case study in Shenzhen.
- Corresponding Author:
- Tongxin Wu
Department of Medicine,
Pioneer Academics,
Philadelphia,
United States
Received: 21-Oct-2022, Manuscript No. JOCPR-22-77986; Editor assigned: 24-Oct-2022, PreQC No. JOCPR-22- 77986 (PQ); Reviewed: 10-Nov-2022, QC No. JOCPR-22-77986; Revised: 17-Nov-2022, Manuscript No. JOCPR- 22-77986 (R); Published: 24-Nov-2022, DOI:10.37532/0975-7384.2022.14(10).017.
Abstract
Carbon dioxide is one of the primary causes of the abrupt rise in global temperature. Researchers are looking for solutions to maintain or lower the global carbon emission level in the current era. Because carbonate (CO3) formed as a result of mineralization is safe and prevalent in Earth crusts, Carbon Capture and Storage (CCS) with mineralization safely tackles the rising carbon emission. In which, amine solutions are introduced and accelerate the conversion. However, the amine regeneration results in high operational costs and energy penalties in traditional methods. The addition of Ca(OH)2 into the solutions can address this problem and has a conversion rate as high as 97.4%. The lower costs and increasing effectiveness through adding Ca(OH)2 will make fossil-fuel-based CCS more competitive. Future breakthroughs are still needed to make CCS cheaper and provide stable renewable energy supplies, enabling carbon neutrality to be more promising.
Keywords
Carbon sequestration; Amine solution; Calcium hydroxide; Fossil-fueled carbon capture; Solar power; Solar carbon sequestration; Solar power price; Single-Process carbon capture; Direct precipitation
Introduction
Carbon Capture and Storage (CCS)
The issue of Climate change has received considerable critical attention in contemporary life: The average temperatures over the next decade will only get higher. In prediction, during the 2050s and 2080s, minimum and maximum temperatures will increase gradually between 0.2 and 2.6°C [1]. Rising sea levels, shrinking glaciers, and increasing temperature are all results of climate change. After decades, evidence suggests that excess carbon dioxide emission is among the most important factors of climate change. Due to intense reliance on industrial progress, the carbon dioxide concentration in our current atmosphere is increasing dramatically. Therefore, scientists are seeking and implementing proper actions toward the excess CO2 in the atmosphere [2].
Carbon Capture and Storage (CCS) technologies can play a pivotal role in addressing the issue of excess CO2 in the atmosphere, through which the carbon level can be diminished effectively both from industrial sources and power plants in industrial developments [3]. The CCS includes various capture methods that directly capture CO2 from factories. The most typical of these methods are air capture, mineralization, pre-combustion, and post-combustion [4].
CaCO3 in Mineralization
Mineralization, the least risky, causes less harm to the environment in the CCS process than other methods. Similar to most CCS techniques, mineralization keeps carbon in a safe place (mostly underground), where it cannot escape back into the atmosphere. Meanwhile, CO2 converts into a solid mineral, Carbonate (CaCO3). Mineralization of CO2 turns out to be more secure and can keep CO2 in a more stable form for storage, benefiting the environment. The properties of CaCO3 posts little harm to both human beings and nature [5].
Calcium carbonate is naturally occurring and a common mineral on earth that makes up 4%-8% of the earth's crust. Compared to the amount of CaCO3 generated from the carbon capture process, the mass does not have a visible effect on Earth and human beings, because of the huge quantity of CaCO3 already existing in the crusts. Therefore, it is reliable to use the carbon precipitation method to capture CO2 as the process does not add up the calcium carbonate deposit largely compared to those already present in the crusts. Additionally, CaCO3 can be widely seen in the ocean where the dead sea animals’ skeletons or shells will accumulate to form CaCO3 [5].
There is a large amount of CaCO3 present in plastic, paper, and other industrial materials. To be more specific, using ground CaCO3 particles to be the filler material for plastics will reduce the material costs in the manufacturing process. When CaCO3 is precipitated and gathered, the particles can become finer and can be applied in more types of industries as nanocomposites [6]. However, carbon dioxide cannot be easily converted into carbonate because of its chemically double covalent bonds as shown in Formula 1 [7]. For this reason, the bonds require the conversion of CO2 to CaCO3 to utilize additional energy inputs.
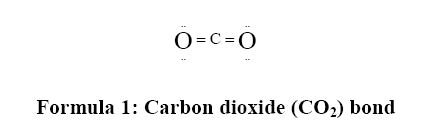
Amine Solution
Catalysts or absorbents are a way to let the reaction happen as shown in Equation 1, an aqueous amine solution is the most known and used absorbent for the carbon mineralization process. The amine solution assists to increase the concentration of the CO2 collected from the atmosphere to make the storage process more efficient [4].
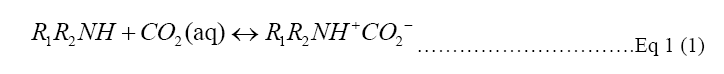
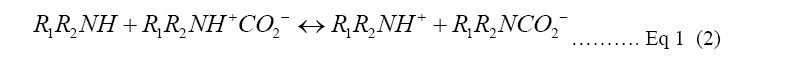
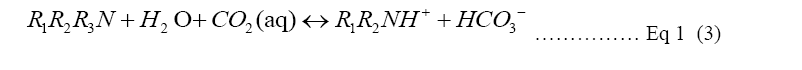
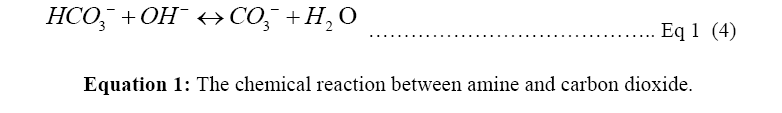
Conversion process of bicarbonate to the final product carbonate through ionization [8].
Materials and Methods
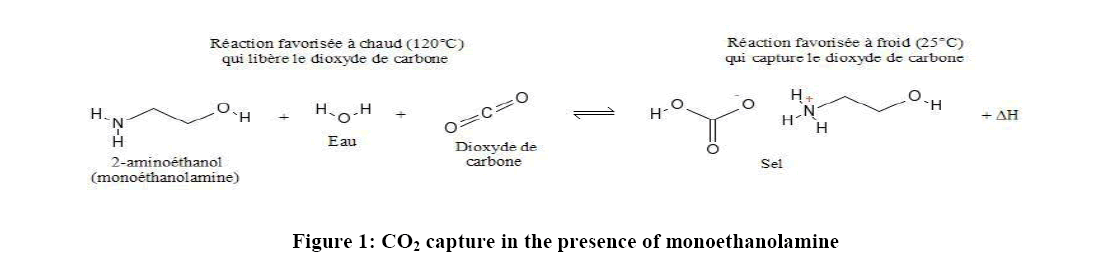
Monoethanolamine (MEA) is a primary amine and one of the R1R2NH used in Equation 1. In a two-step reaction, primary and secondary alkanolamines react with dissolved CO2, forming first a zwitterion (1), then protonating an unionized amine, producing carbamate (2). In the reaction of tertiary amines with CO2, a protonated amine and a bicarbonate anion are formed (3) [9,10]. The reaction of MEA with H2O and CO2 to form pure carbon dioxide for recollection into amine solution is shown in Figure 1. Monoethanolamine (MEA) here is used as an example of the Primary alkanolamines reacting with dissolved CO2 in two step reactions [9].
Amine solution is usually used as an effective mineralization carbon capture base for CO2 to convert into a safer and stable Carbonate. The process is illustrated in Equation 1. Collecting the bicarbonate HCO3- in the third step in Equation 1, CO3 can be gotten through HCO3- ionizing with H2O as shown in step 4. Considering the effectiveness of aqueous amine absorbent, the disadvantages may cause severe environmental and economic problems [3]. As current amine solution has a relatively low CO2 absorption rate to make the solution reach an optimal CO2 loading concentration, it requires energy for regeneration of the amine to capture more amount of CO2 from the atmosphere.
Regeneration in Traditional CCS
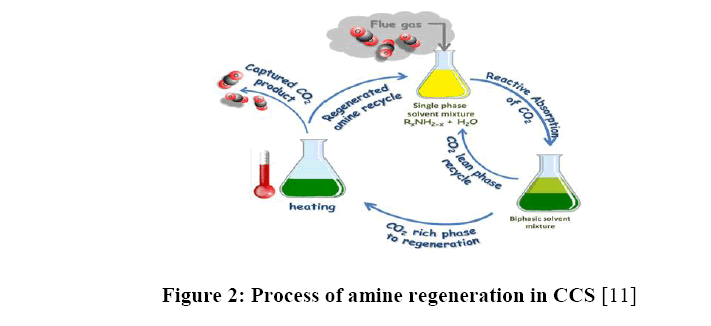
The traditional Amine-scrubbing process is shown in the Figure 2. The yellow solution indicates the initial amine solution. When the flue gas (CO2) from factories, for example, is absorbed into the amine solution, the CO2 rich phase (high concentration of CO2) and CO2 lean phase (low concentration of CO2) proceed into two pathways. The former enters the regeneration process where the solution is heated to generate more space for absorbing more CO2 for the next round because of the inefficiency of the traditional CCS. The latter one will stay in the solution in ideal situations. Some of the CO2 will escape into the atmosphere inevitably while the regenerated amine solution is capturing another turn of CO2 [11]. The reheating and the CO2 escaping emphasize the importance of developing a more effective CCS method.
Figure 2: Process of amine regeneration in CCS [11]
Energy penalty: Energy Penalty is a parameter to check how efficient the CCS process is. To produce a fixed quantity of work output, a fixed fraction of fuel must be devoted to CCS. Depending on the fuel input, the penalty can either be an increase in fuel consumption or a loss of output [12]. The traditional method of amine solution regeneration has an energy penalty of 40% and consumes around 70% of the total energy used for the whole carbon capture process [13,14]. Researchers discovered that most amine solutions are regenerated best under the temperature of 120°C, while the operation and capital cost for the heat regenerating (thermal regeneration) of these amine solutions can be enormous [3]. The high energy penalty is resulted from the fact that the entrance of CO2 into the solution is an in-spontaneous reaction (ΔG°reaction > 0) where it requires more energy for CO2 to start the precipitation process, but the diffusion of CO2 from the solution to the atmosphere is a spontaneous reaction where less energy is required by CO2 for this process. Thus, additional energy, from heat, is indispensable for amine regeneration [15].
CCS efficiency improvement technologies: Taking this problem into consideration, potential improvements have been made. Some studies suggest using renewable energy with less energy penalty, including switching to solar energy. Other studies aim to find substituted absorbents of amine solution while conducting mineralization. Yet, the key to reducing the cost is to decrease the frequency of thermal regeneration of amine solution before any other promising solutions have been developed. In the investigation by Kang [3], the single process CO2 precipitating approach represents its efficiency and usefulness in reducing the high capital of carbon capture, which makes the use of heat for regeneration to be reduced.
Direct precipitation with calcium hydroxide: The paper is also going to discover the effect of direct CO2precipitation. The single process precipitation of CO2 means capturing carbon not with regenerating amine solution but by directly precipitating carbon dioxide by introducing Ca(OH)2 with a conversion rate of 97.4%. The high conversion percentage with this method will decrease the need for regenerating amine and save energy at least by 40% [16]. The overall concept is based on the natural carbonation depending on the acidity of water in a slow process. As shown in Equation 2, the calcium carbonate is produced, and the process is similar to the reaction in Equation 1.
The amine solution will be present between steps (1) and step (2) to continue the CCS process as the life cycle of CCS in Figure 2. illustrated [4]. The gaseous state carbon dioxide turns into aqueous carbon dioxide first and to bicarbonate. Finally, the carbonate combines with calcium from calcium hydroxide to become calcium carbonate [16]. Without additional steps of amine regeneration, the capital and operational costs would inevitably become lower and the process will accelerate the carbonation dramatically compared to the current CCS method [3,16].
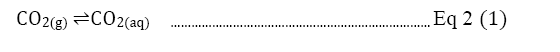
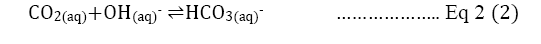
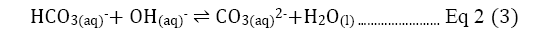
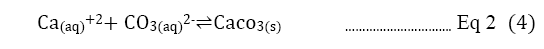
Equation 2: Listed by illustration of how carbon dioxide turns into calcium carbonate with the reaction of hydroxide [17].
Competitiveness of fossil-fuel energy supply for CCS: Subsequent to various variable control tests of the single process carbon capture and mineralization, the single process CCS has been testified. When a large amount of electricity cost for generating the heat for amine regeneration is minimized, the CCS benefiting the environment and humans can be implemented more practically. Due to the instability of solar power, it is unpromising to use solar power to conduct CCS, despite its current lower electricity price. It is also impractical to wait until a stable solar power supply is available to continue carbon capture (Equation 3).
Yet, citizens may not think about the application possibilities. The problem is that at a lower price, they would rather not invest in CCS than in something that costs more than Solar Power: There is a 0.27 CNY/kwh price discrepancy between fossil-fueled energy and solar energy in Shenzhen, China. Both solar power and fossil fuel-based CCS aim to achieve carbon neutrality. The lower price of running fossil fuel-based CCS thanks to direct precipitation with the addition of Ca(OH)2 may capture citizens’ attention more on fossil fuel-based CCS than on solar CCS.
Fossil-fueled CCS technologies, and direct CO2 precipitation, may in turn have more supporters to promote further advancement. The paper examines existing CCS breakthroughs with a decreased price that may compete with the predicted solar CCS. Additionally, the paper will calculate the CO2 desorption rate of the direct precipitation and compare the electricity prices of fossil-fuel-based and solar-based electricity.
The comparison will specify how important the single process CCS is an estimate how the direct capture CCS price will switch the future preferences in carbon capture energy supply types. More investment and emphasis can be placed on fossil-fuel CCS without worrying about its higher operating costs than solar CCS.
Results
Single process precipitation presents its promising aspect: Prove its efficiency by determining the CO2 absorption rate under the introduction of various amine absorbents, including Monoethanolamine (MEA), Diethanolamine (DEA), N-Methyldiethanolamine (MDEA), and 2-Amino-2-Methyl-1-Propanol (AMP) [16]. The main function of amine solution in the process of carbon capture is to carry the CO2 and avoid CO2 escaping into the atmosphere as much as possible. These trials evaluating different amine test which adsorbent is the most effective in regeneration.
To measure the effectiveness of CCS technologies, the CO2 Desorption rate is taken into account to calculate the efficiency of carbon capture using amine loading. The desorption rate is essential to unbind the CO2 from the amine solution. The release of CO2 into the atmosphere helps the solution gather more CO2 for the next round of amine scrubbing (CO2 absorption and desorption with regeneration). Amine loading, i.e., the amount of CO2 captured per mole of amine, is one of the parameters to calculate the efficiency where CO2 adsorbs with an amine solution to be stored in the solution without further posing an impact on the atmosphere.
To make the amine loading usable for the CO2 Desorption efficiency equation, rich amine loading and lean amine loading should be figured out in Equation 4. [16]. The concept of lean amine loading indicates amine regeneration efficiency and CO2 absorption capacity. A rich amine load determines its maximum CO2 absorption capacity [18].
In the new proposal Ca(OH)2 introduction eliminates the frequent regenerating process of amine solution. Principally, the addition of OH increases the pH level, resulting in a higher carbon desorption rate [3]. As Equation 3 Shows, the calcium ions connect with protons from bicarbonate (HCO3) ions to create carbonate (CO3) ions which can bond with Ca [19].
In the presence of calcium carbonate, the pH of the solution increased until all calcium and bicarbonate ions were reacted, which maximizes the carbon concentration and curtails the need of thermal regeneration. Moreover, Ca(OH)2 is formed from CO2 when it reacts with Ca2+ thereby securing Ca(OH)2 for the long term for creating CO3 [16].
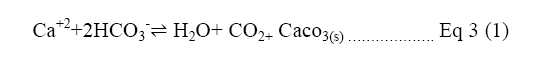
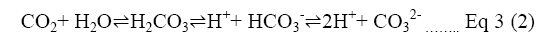
Equation 3: Formation of CO3 from HCO3 ions where HCO3- indicates bicarbonate; CaCO3 indicates calcium carbonate; H2CO3 indicates carbonic acid; CO3 is the final produce carbonate
Increased CCS efficiency using direct precipitation should be considered through checking the CO2 desorption efficiency calculation formula as below. Listed below are the CO2 Desorption rates for traditional methods and single process carbon capture, which show that adsorption of carbon dioxide by a single process is more efficient, especially when carbon is left in the solution 10 times less than it was with a traditional process [16].

The traditional thermal treatment approach after the precious amine-scrubbing process is usually performed by heating the amine solution under the temperature 90°C-120°C. In Equation 4, the rich and lean amine loading is taken from the reheated amine solution at 90°C. In the trial of alternative mineralization CCS, tested the regenerating procedure of thermal treatment, lean amine loading is lacking in order to perform a complete calculation of CO2 desorption rate [16].
According to the calculation process provided in Equation 4, it turns out that the lean amine loading is 0.077 mol CO2 per mol of amine solution (by setting the initial lean amine loading as a variable X and plugging the X in the equation to start the computation). The significance of this number can be determined through the comparison of rich amine loading and lean amine loading of the single process mineralization of CO2 illustrated by Heldebrant, et al. and Li, et al. [20,21].
The reason to overturn the thermal amine regeneration is that the energy penalty for the traditional amine scrubbing is too high to make the carbon capture process useful. Based on the practical cases, the energy penalty values for 100% capture amounted to 48.6% for liquefied and 43.5% for compressed [22]. Almost half of the energy spent on heat emphasizes that an improved cost-effective system needs to be developed. The high electricity output also contributes to the release of CO2 into the atmosphere.
Carbon Desorption Efficiency of Single Process CCS
In the experiment, amine absorbents are tested to conduct the integration of CO2 absorption and mineralization to reduce the energy required for reheating the amine solutions to increase their CO2 loading. The Murnandari group tested Monoethanolamine (MEA), Diethanolamine (DEA), N-Methyldiethanolamine (MDEA), and 2-Amino-2- Methyl-1-Propanol (AMP) with the thermal regeneration method to show the most effective amine solution in the process [16]. These amines are also represented as R1R2NH, R1R2R3N, and R1R2R3NH+ in the introduction. The efficiency of each amine absorbent is listed below as performed.
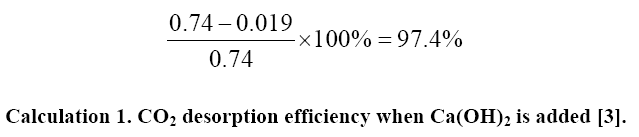
Comparing the absorption rate and desorption rate, the trend that a higher absorption rate may cause a lower desorption rate is shown in Table 1. MEA solution turns out to have the highest CO2 absorption rate and the lowest desorption rate, while MDEA solution has the lowest absorption rate with the highest desorption rate. In the example of AMP solutions which have the highest working capacity, the rich amine loading is 0.74 mol of CO2 per mol of amine and the lean amine loading is 0.22 mol of CO2 per mol of amine [16].
| Method | Thermal treatment | |||
|---|---|---|---|---|
| Amine | Â MEA | DEA | MDEA | AMP |
| Rich loading (mol of CO2/mol of amine) | 0.66 | 0.68 | 0.7 | 0.74 |
| Lean loading (mol of CO2/mol of amine) | 0.44 | 0.19 | 0.2 | 0.22 |
| Absorption rate (mmol mol-1s-1) | 0.204 | 0.2 | 0.1625 | 0.1916 |
| Desorption rate (mmol mol-1s-1) | 0.0542 | 0.108 | 0.138 | 0.083 |
| Working capacity (mol of CO/mal of amine) | 0.22 | 0.49 | 0.5 | 0.52 |
Table 1: Comparison between various amines? efficiency using thermal treatment [16].
In the experiment done the CO2 conversion efficiency with the addition of Ca(OH)2 is 97.4%, and the amine regeneration efficiency is calculated to be 0.019 mol CO2/mol amine [3]. The result of 0.019 mol CO2/mol amine is figured by hypothesizing the amine solution’s maximum absorption capacity is the same as that of the AMP solutions. In Kang's experiment, the lean amine loading would be smaller regardless of which rich amine loading in Table 1. Was used in the calculation. This is because of the negative linear relationship between rich amine loading and lean amine loading which proves the technique studied by Kang to be more effective. Calculation 1 is an example of a process in which Kang and their group receive a CO2 desorption efficiency of 97.4%.
The percentage is significant as it restores the calculation process used by the Kang et al. in arriving at their final CO2 conversion rate (an achievement of CCS) [3]. Also, the complete conversion percentage is 30% greater than the conversion rate of the traditional amine regeneration method, largely showing the cost-saving and effectiveness of the single process precipitation method. Conversion rates as high as 97.4% reduce the need to regenerate and recollect CO2 from the atmosphere, saving money and electricity cost [3,23].
Besides, comparing the lean amine loading of the traditional CCS method with that of the single process CCS with AMP solution, there is a 0.143 mol of CO2/mol of amine divergence. The lean amine loading of AMP solution (0.22 mol CO2/mol amine) and the lean amine loading when Ca(OH)2 is added (0.019 mol CO2/mol amine). Considering the negative correlation between rich amine loading and lean amine loading, the lean amine loading difference in Table 1. and Calculation 1. indicates that adding Ca(OH)2 into the amine solution in the CCS process can maximize CO2 absorption capacity. Therefore, the direct precipitation with Ca(OH)2 allow the technology to be more efficient.
The Application of Calcium Hydroxide in CCS
The data obtained from the document comes from calculating the direct precipitation of carbon dioxide by introducing Ca(OH)2 at 40°C [3]. Different from testing the efficiency of various amine absorbents conducting thermal regeneration, Ca(OH)2 is added to the solution to test the conversion rate of CO2 to CaCO3 by weighing the mass of CaCO3. After CaCO3 forms in the amine solution, the CaCO3 would be collected and dried in an oven at 60℃ for one day. Ca(OH)2 would be added twice into the amine solution to promote the conversion, and the CaCO3 would also be collected twice. The dried CaCO3 mass then would be calculated to get the percentage of 97.4% (CO2 desorption rate). The 97.4% conversion rate through this process is extraordinary, especially when the required temperature for the Ca(OH)2 carbon capture process is only 40°C and 60°C for oven drying compared to 90°C in the traditional thermal regeneration [3].
The lower temperature needed for the regeneration after adding Ca(OH)2 is because of Le Châtelier's principle: Whenever a dynamic equilibrium is disturbed by a change in conditions, including heat, the equilibrium position shifts to compensate for the change [24]. According to the Ca(OH)2 solubility experiment done by an increase in the temperature of a supersaturated Ca(OH)2 solution will decrease the activity of OH– ions, shown in Table 2 [25-27]. The higher temperature hinders the solubility of the Ca(OH)2, and the additional higher temperature will in turn shift the solubility of Ca(OH)2. As a result, a lower temperature may maintain the solubility of Ca(OH)2 in the solution to an equilibrium rate to save heating costs. This characteristic of Ca(OH)2 may benefit the CCS process and should be used widely as it does not require a high temperature for thermal regeneration and does decrease the frequency of amine regeneration for its high amine loading level [3,16].
| No. | Temperature (°C) | Co pH Solubility | Ca(OH)2/100 ml H2O. gr. |
|---|---|---|---|
| 1 | 20 | 12 | 0.173 |
| 2 | 50 | 11.5 | 0.147 |
| 3 | 70 | 11.3 | 0.115 |
| 4 | 100 | 10.8 | 0.09 |
Table 2. The relationship between temperature and solubility of calcium hydroxide is measured with an activity of OH? ions
Scaling Up to Field Scale
Energy penalty improvements: Scaling up to field scale is necessary as it makes the single process carbon capture go beyond the lab environment and capture the carbon in the wild. Besides the improved CCS efficiency based on the breakthrough in the single process precipitation, cost, and energy penalties are also reduced in the application. Energy penalty is an important factor in considering the usefulness of single process amine-scrubbing as the needed energy penalty is 48.6% for traditional ways of liquifying CO2 for a 100% capture rate calculated with Equation 5. However, the direct precipitation reduced the needed energy to run the CCS by 22%, which means to reduce the energy penalty for liquified CO2 to 26.6% from previous 48.6%. The single-process CCS testing shows energy savings of 2%-7% for MEA and DEA, suggesting the tested amine system is more effective and durable [3,22].
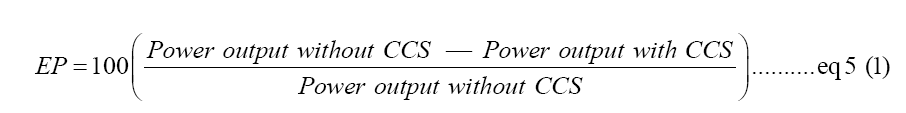
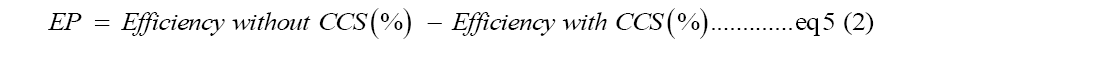
Equation 5: An evaluation of the impact of adding CCS to a power plant. This literature frequently quotes energy penalty values [22].
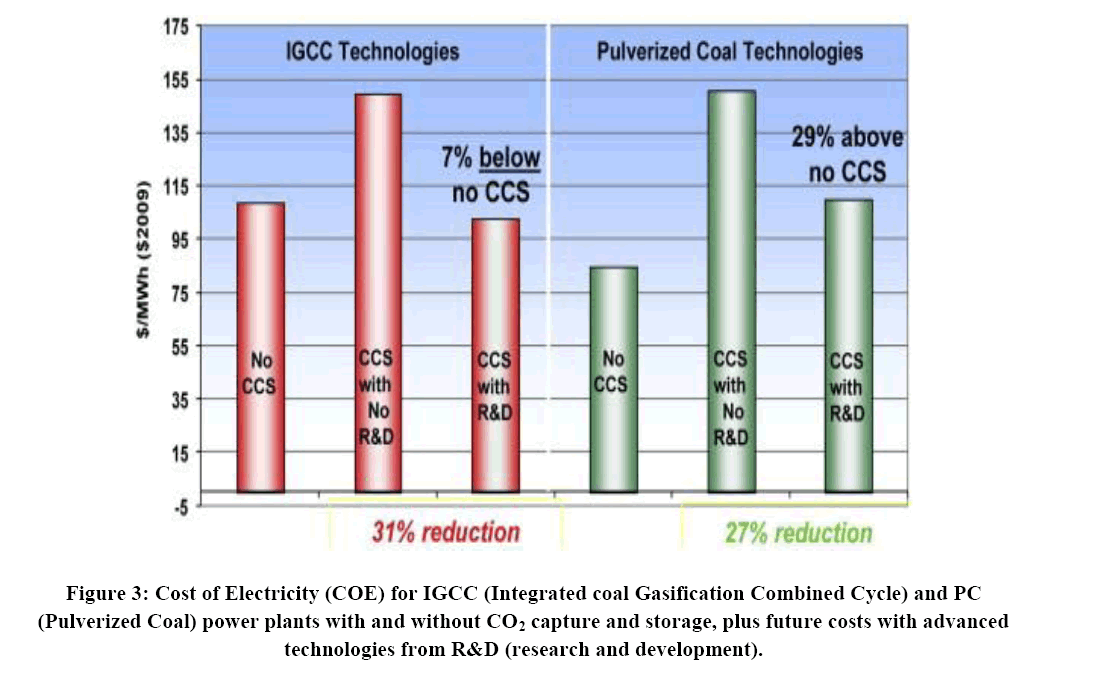
Cost improvements: In Figure 3, the Costs of Electricity (CoE) for Pulverized Coal plants (PC) and Integrated Gasification Combined Cycle (IGCC) power generations are illustrated. PC plants use the coal to mix with air burning in the furnace to produce heat for regeneration, also known as the post-combustion process, while IGCC plant refers to heat coal with steam and oxygen at high temperature and pressure naming gasification in order to remove carbon from the fuel prior to combustion-known as pre-combustion processes [3,4].
Case Study of CCS in Shenzhen
In the highly-developed city of Shenzhen, China, the fossil fuel electricity price was gradually decreasing from 4 CNY/kwh in 2007 to 0.7 CNY/kwh (0.699 CNY/kwh) in 2018 (all taxed) according to China Southern Power Grid. The progress over 12 years promotes a 92% decrease in electricity prices. The electricity cost is excerpted from the last roll of the grid for residents or households who have not yet adopted the “one household, one-meter” implementation, indicating that many neighbours share the same meter and split the electricity bill according to the price of the same meter [23]. Integrated meter electricity price is short for residential integrated meter user electricity price. It is a kind of electricity price that is measured by multiple users sharing one electricity meter and cannot be measured by the stepwise electricity price. Because the total meter price indicates the average electricity price, it is used for the following case study calculation.
Potential of the COE Decrease on Fossil-Fuel-Based Carbon Capture
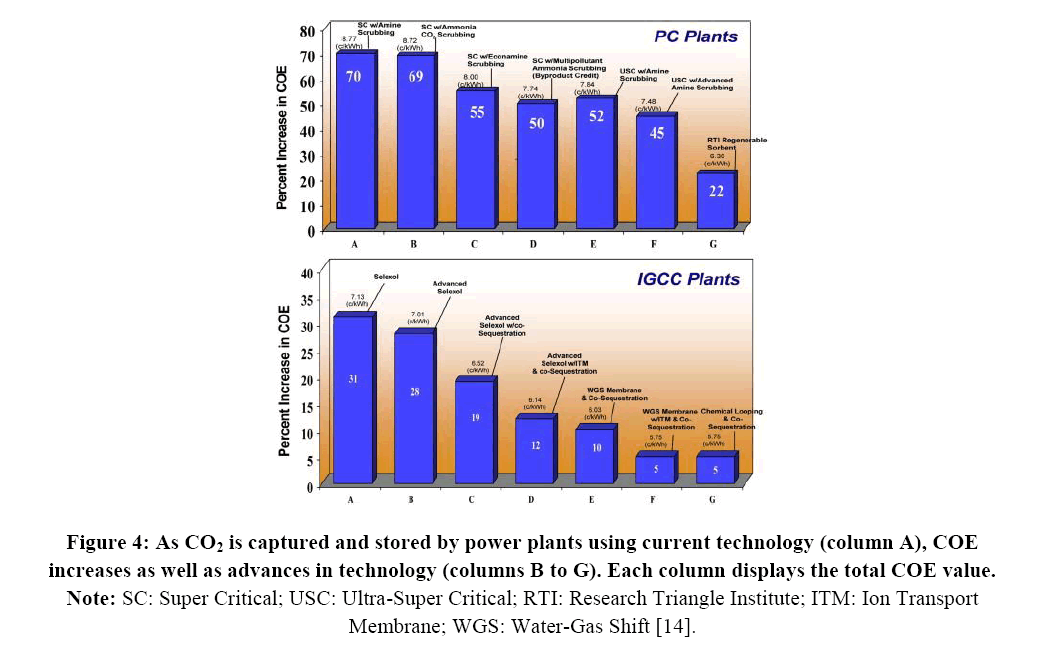
With the conduction of carbon capture in Shenzhen to achieve carbon neutrality, also applied to other areas, the cost of energy will increase according to certain CCS technologies being used. In Figure 4, the COE increases from 22% to 70% for PC Plants. In the case of Shenzhen as shown in Table 3, the fossil fuel COE would have an increased range from 0.854 CNY/kwh to 1.19 CNY/kwh for PC plants. On the other hand, the range for fossil fuel COE would be 0.735 CNY/kwh to 0.917 CNY/kwh for IGCC plants [28].
| Shenzhen residents living electricity price list (Effective from February 1, 2022) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Â Unit: cent/kwh (tax included) | ||||||||
| Classification of electricity consumption | Standard electricity price | Total additional funds | National major water conservancy project construction fund | Â Late support fund for large medium-sized reservoir settlement | Support fund for small scale reservoir resettlement in later period | Total electricity cost | ||
| Residential household electricity price | Step tariff | First gear (0-260 degrees in summer, 0-200 degrees in non-summer) | 65.42 | 0.8669 | 0.19688 | 0.62 | 0.05 | 66.286875 |
| Second gear (261-600 degrees in summer, 201-400 degrees in non-summer | 70.42 | 0.8669 | 0.19688 | 0.62 | 0.05 | |||
| Third gear (above 600 degrees in summer, above 400 degrees in non-summer | 95.42 | 0.8669 | 0.19688 | 0.62 | 0.05 | |||
| Peak to valley tarrif (first determine time used, then calculate step tarrif) | Â Peak (10:00-12:00, 14:00-19:00) | 111.21 | 0.8669 | 0.19688 | 0.62 | 0.05 | ||
| Â Flat period(8:00-10:00, 12:00-14:00, 19:00-24:00) | 65.42 | 0.8669 | 0.19688 | 0.62 | 0.05 | |||
| Trough period (0:00-8:00) | 24.86 | 0.8669 | 0.19688 | 0.62 | 0.05 | |||
| The second incremental electricity price (261-600 degrees in summer, non-summer season 201-400 degrees) | 5 | 5 | ||||||
| Third grade incremental electricity price (above 600 degrees in summer above 400 degrees in non-summer) | 30 | 30 | ||||||
| Total meter price | 69.12 | 0.8669 | 0.19688 | 0.62 | 0.05 | |||
Table 3: The translated version of Shenzhen residents living electricity price with total meter price and different calculation categories. Table created by China Southern Power Grid, G. in 2022 [23]
Figure 4: As CO2 is captured and stored by power plants using current technology (column A), COE increases as well as advances in technology (columns B to G). Each column displays the total COE value.
Note: SC: Super Critical; USC: Ultra-Super Critical; RTI: Research Triangle Institute; ITM: Ion Transport Membrane; WGS: Water-Gas Shift [14].
For solar energy, the price is at an average of 0.5 CNY/kwh and sometimes with government subsidies down to 0.42 CNY/kwh nowadays [23]. We can predict (no solar CCS available currently due to the instability of solar power supply) that the solar COE with CCS on PC plants would have an increased range from 0.512 CNY/kwh to 0.85 CNY/kwh for PC plants.
The range for solar COE with CCS on IGCC plants will be from 0.441 CNY/kwh to 0.655 CNY/kwh. Even with the progress of setting up more power stations in China, solar energy still has a lower price and in turn, is preferred by most of the citizens. The lower price of solar electricity distracts people’s attention from fossil-fueled CCS because citizens are hoping for lower-priced solar CCS, even though the solar power is not stable enough for the CCS implementation.
To continue promoting the development of CCS, lower COE for stable fossil-fueled CCS than that for solar CCS may erase citizens' and investors’ concerns about CCS price. When the fossil-fuel-powered CCS becomes cheaper, more funds and improvements on current carbon capture technologies will be present. The price drop is especially important when a stable supply of solar power is still far from achieving, and the improvement of CCS can reduce the risk of CO2 in the atmosphere causing further global warming.
Reflecting on divergent CCS technologies around the world, the total costs saves a different percentage of energy. A Metal-Organic Framework (MOF)-based filter along with vacuum Pressure Swing Adsorption (PSA) technology can prevent 80% of the predicted total CCS running costs [29,30]. As CycloneCC (one of the CCS improvements) indicated, 50% of capital and operational expenses will be saved. Plus, a diamine-derived CO2BOL N-(2- ethoxyethyl)-3-morpholinopropan-1-amine (2-EEMPA) shows its effectiveness in carbon capture by reducing the total costs by 19% from the original $58/ton to $47.1/tonne CO2 [31]. These existing carbon capture technologies present the potential for direct precipitation and other CCS with R&D.
Goals for future CCS: According to the fossil fuel price of electricity in Shenzhen, the cost of electricity will be cut from 1.19 CNY/kwh (70% increase in COE of PC plants) to 0.238 CNY/kwh if 80% of total costs can be decreased; 1.19 CNY/kwh will reduce to 0.595 CNY/kwh if 50% of total costs can be reduced; and to 0.9639 CNY/kwh if 19% of decrease in total costs is plausible. As stated in Equation 6, compared to the hypothetical lowest solar energy price with government subsidies of 0.441 CNY/kwh, the COE with CCS has to be more competitive in order to win the public’s attention to support CCS [23]. In the future, the CCS improvements can aim to decrease the CCS total cost by 63% as shown in Equation 6, so that fossil-fuel-based CCS can compete with solar CCS. The data will be explained further in discussion. Instead of waiting for cheaper solar power CCS energy alternatives, actions should be made immediately to provide price advantages in fossil-fuel-based-CCS.
Predicted Lowest Solar COE with CCS
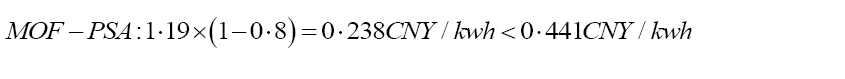
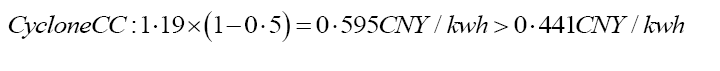
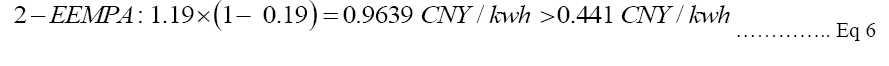
Equation 6: Price discrepancies of the three different CCS technologies compared with the current solar cost of electricity.
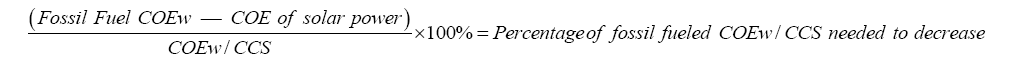
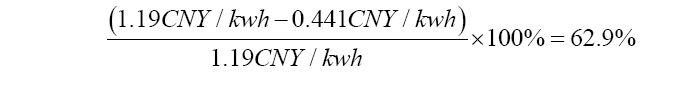
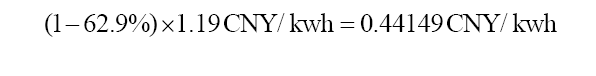
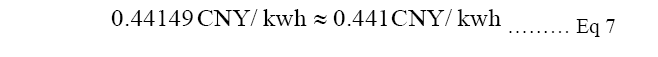
Equation 7: The equation calculates when the COE of fossil fuel power will be cheaper than COE of solar power to be more competitive. At least a 64.7% decrease by technology improvemetns of CCS will the advancement is effective.
Discussion
In the case study of Shenzhen, the fossil fuel electricity price per kilowatt per hour is 0.7¥ compared to 0.42¥ for solar energy [23]. Compared to the price divergences between different CSS technologies, less energy required for CCS is ideal for saving operational costs and energy output. The lower required energy can result from less frequency of amine regeneration and reduced temperature for amine scrubbing, emphasizing the significance of the direct precipitation CCS method.
Even though some of the existing CCS technology may post some price advantages, 50% of the total cost decrease of CCS with R&D still has a 0.175 CNY/kwh discrepancy compared to the pure solar price (Figure 3). In the future, the plausible application of the direct precipitation of Ca(OH)2 needs to pose a larger price advantage for people in order to make a less stable CSS using solar energy less tempting and cheaper than CSS with fossil fuel energy after the development. In estimation, if the cost of fossil fuel electricity decreased by 63% consistently for most CCS advancements as shown in Equation 7, the single process carbon capture may be able to compete with future CSS with solar power. After the problem of unstable solar energy supply is addressed, it may cost no less than 0.417 CNY/kwh in the CCS process (IGCC plant), while solar power itself still costs 0.42 CNY/kwh. Not until then, the carbon emissions energy cost may still need effective solutions to be reduced and supported to make the CSS process more secure, and people may still believe that solar energy is the best way to conduct CSS considering its lower costs and less energy penalty.
If the COE with CCS can decrease by 63% depending on the technology improvements, the carbon capture will garner more attention and have the potential to be used outside the laboratory settings. Therefore, it is important for both the public and researchers to put more emphasis on new fossil-fuel-based CCS breakthroughs. In the section calculating the carbon dioxide desorption rate, it is concluded that single process carbon capture with calcium hydroxide is promising. CCS is worth noticing because it solves the most pivotal “high regeneration energy” problem and sequesters carbon dioxide with a higher loading level. If the Ca(OH)2 introduction provides a positive cost feedback, future research may need to focus on diffusing Ca(OH)2 in fields with various methods and testing trials.
In future work, investigating the extraction of calcium hydroxide from industrial waste in order to apply industrial CCS is required. Also, how to spread amine solution with Ca(OH)2 on a larger scale, in natural circumstances, is a problem. If the solution is figured out, the potential of future carbon capture and storage applications is unimaginable, especially when the price is no longer an obstruction, and the excess carbon dioxide in the atmosphere can be converted into a harmless, stable form of CaCO3 more readily.
Conclusion
The current enormous energy penalty for the traditional amine regeneration carbon capture method contains a percentage close to 50% for 100% carbon capture: Amine solution requires laborers to reheat the solution to release and capture a higher concentration of carbon dioxide and needs high operational and capital costs. The inefficiency is represented not only through the low amine loading for the traditional ways but also through the relatively more expensive fossil fuel electricity used in the process. In order for carbon capture mineralization to become more widespread either in factories or in larger fields, more efficient carbon capture technologies must be developed. Currently, the direct carbon capture concept designed by adding Ca(OH)2 to increase the amine loading per mole of amine is testified to reduce the energy penalty and capital cost. However, one of the impediments to further developments on fossil-fueled CCS is citizens’ increasing attention to the cheaper, less stable solar power CCS. Promisingly, this issue is likely to be addressed with the lower COE on fossil-fuel-based CCS designed by future single-process precipitation technologies. Meanwhile, the public and researchers should place more emphasis on fossil-fuel-based CCS breakthroughs, aiming at reducing the fossil-fueled CCS COE by 63%, instead of being distracted by substitutes, solar CCS, for the fossil-fuel-based CCS and interrupting the breakthroughs of CCS.
References
- Shagega FP, Munishi SE, Kongo VM. Phys Chem Earth. 2019;112:3200–209.
- Fleming RJ. J Environ Manage. 2018;77(6):1-3.
- Kang JM, Murnandari A, Youn MH, et al. Chem Eng J. 2018;335:338-44.
- Kumar H, Ravikumar S. Adv Space Res. 2010; 13:364-71.
- Calcium carbonate. 2022.
- Lin Y, Chan CM. Woodhead Publ. 2012.
- Paparo A, Okuda J. Coord Chem Rev. 2017;334:136-49.
- Which formula can be used to calculate the exact hydronium concentration present in sodium.2018.
- Nasa Space. 2022.
- Halme A. Biol. Futura. 2022.
- Papadopoulos AI, Tzirakis F, Tsivintzelis I, et al. Ind Eng Chem Res. 2019;58(13):5088-111.
- House KZ, Harvey CF, Aziz MJ, et al. Energy Environ Sci. 2009;2(2):193-205.
- Hamdy LB, Goel C, Rudd JA, et al. Adv Mater. 2021.
- Rao AB, Rubin ES. Environ Sci Technol. 2002;36(20):4467-75.
- Hills CD, Tripathi N, Carey PJ. Front Energy Res. 2020;8(8).
- Murnandari A, Kang J, Youn MH, et al. Korean J Chem Eng. 2017;34(3):935-41.
- Arti M, Youn MH, Park KT, et al. Ener Fuel. 2016;31(1):763-9.
- AFPM. 2022.
- Ocean Chem Acid. 2021.
- Heldebrant D, Jiang Y, Zheng R, et al.SSRN Electronic Journal. 2021.
- Ji L, Li J, Liu N, et al. Greenh Gases: Sci Tech. 2022;12(4):508-19.
- Page SC, Williamson AG, Mason IG. Energy Policy. 2009;37(9):3314-24.
- China Southern Power Grid. 2022.
- Libretexts. 2020.
- Luis P. Energy Procedia. 2009;1(1):791-7.
- Saipullaev M, Koichuev A, Batyrova A, et al. E3S Web Conf. 2020.
- Skocek J, Zajac M, Ben Haha M. Sci Rep. 2020;10(1):1-2.
[Cross Ref] [Google Scholar] [Pub Med]
- Rubin ES, Mantripragada H, Marks A, et al. Prog Energy Combust Sci. 2012;38(5):630-71.
- Wright A. Gasworld. 2022.
- Van Der Spek M, Arendsen R, Ramirez A, et al. Int J Greenh Gas Control. 2016;47:176-99.
- CYCLONECC™: The Future of Carbon Capture.2022.