Reviews: 2022 Vol: 14 Issue: 6
CeO2@Xanthan Ncs as an Efficient Green Nanocatalyst for Removal of Some Environmentally Poisons
Karim A. Hamdhaei*
Department of Research and Development, Hormozgan University of Medical Sciences, Bandar Abbas, Iran
- Corresponding Author:
- Karim A. Hamdhaei
Department of Research and Development
Hormozgan University of Medical Sciences,
Bandar Abbas,
Iran
Received: 3-Mar-2022, Manuscript No. JOCPR-22-49728;Editor assigned: 7-Mar-2022, PreQC No. JOCPR-22-49728 (PQ); Reviewed: 21-Mar-2022, QC No. JOCPR-22-49728; Revised: 2-May-2022, Manuscript No. JOCPR-22-49728 (R);Published: 17-May-2022, DOI: 10.37532/0975-7384.2022.14(5).003
Abstract
During this study, a potent bioactive CeO2@Xanthan nanocomposite (NCs) was green synthesized using the aqueous extract of the Allium tricoccum and characterized using the spectroscopic and surface analysis techniques. The catalytic activity of CeO2@Xanthan NCs was evaluated for the removal of polyaromatic hydrocarbons and conversion of poisonous 4-Nitrophenol (4-NP) to 4-Aminophenol (4-AP). The results demonstrated an excellent bioactivity and superior catalytic activity for the mentioned NCs. Also, the catalyst showed a very good recyclability in considerable numbers of consequent aforementioned reactions.
Keywords
Green CeO2@xanthan NCs; Allium tricoccum; Polyaromatic hydrocarbones; 4-Nitrophenol, Recyclability; Antioxidant
Introduction
Allium tricoccum (Alliaceae) shows a lot of applications in traditional medicine for presence a high concentration of bioactive phytochemicals especially antioxidant polyphenolics in its extract, (Figure 1). Thus, during this study, it was used as an excellent source of antioxidants to biosynthesis of CeO2 NPs through an ecofriendly procedure. Among the most widespread pollutants, Polycyclic Aromatic Hydrocarbons (PAHs) are most toxic molecules found largely in pyrogenic and petrogenic sources such as coal and tar deposits and also incomplete combustion of organic matters. Their characteristics as enviro nmentally persistent poisons converted them as one of the most dangerous threats for living systems especially human life. Therefore, through this research the green synthesized CeO2 NPs as nano adsorbent was employed to removal of aromatic hydrocarbons including PAHs from the real sample of crude oil as model [1].
Stevia rebaudiana belonging to Asteraceae also known as “sweet herb”, a native plant of Paraguay is used as a natural sweetener. Stevia is a perennial herbaceous plant that can grow up to 60-80 cm tall. The leaves of this plant contain glycosides (steviosides and rebaudiosides) which are 100-300 times sweeter than sucrose. The glycoside, rebaudioside a present in stevia is 400 times sweeter than sucrose. One of the major areas of uses of stevia is food supplement for diabetic peoples. There is no permanent drug for the treatment of diabetes and the recent oral hypoglycemic drugs have undesirable side effects on patients. It is proven that stevia controls blood sugar level and it is legally approved as food additives in Brazil, Korea and Japan. But the major limiting factor for the large-scale production of stevia is the low percentage of germination capacity of stevia seeds [2].
Apart from, Stevia possesses some additional properties such as anti-hypertensive, anti-hyperglycemic and anti- human rotavirus. Consuming stevia on a regular basis will not produce any dental caries effect; this was confirmed in a research study. A glucose tolerance test was performed before and after administration of the stevia leaf extracts. The results showed that treatment with Stevia resulted in an increase in glucose tolerance and a decrease in plasma glucose concentrations. And it was shown recently that both steviol and stevioside produce a direct effect on beta cells in the islets of Langerhans of pancreas to release insulin. The authors concluded that this plant may have a potential use in the management of type 2 diabetes. In South America and Japan have a history for the use of stevia sweeteners and there are no adverse effects recorded. And the antioxidant activity of stevia is due to the presence of phenol and flavonoids. Phenols are important in the plant for normal growth and defense against infection and injury. The presences of phenolic compounds in injured plants have an important effect on the oxidative stability and microbial safety. Although phenolic compounds do not have any known nutritional function, they may be important to human health because of their antioxidant potency. Based on available literature report, we opted these two plants [3] from same family (Asteraceae), and compared to estimate total phenolic content, total antioxidant, and DPPH scavenging potential of Achillea millefolium, Stevia rebaudiana ethanolic and aqueous extracts.
Materials and Methods
Nowadays the application of nitro containing compounds is of great interest especially due to their importance in pharmaceutical and nutritional industries. The ever-increasing demand for removing the hazardous compounds in which widely used as a thickening and stabilizing agent in a wide variety of food and industrial [4] samples containing nitro compounds such as 4-nitrophenoles and decreasing their side effects on the ecosystem cased to develop the more efficient and safer methods with respect to the principals of green chemistry. Reducing the agglomeration of nanoparticles due their surface potential is an interest of researchers of the field. To prevent this problem and over-stoichiometric use of metal reagents, application of bio-supports for deposition of metal NPs which provides both a cost-effective and green way for the nanocomposite production is current policy of the researchers. Xanthan gum is a complex exopolysaccharide, produced by plant-pathogenic bacterium products. During this study we employed this bio support to deposition of green synthesized CeO2 NPs and finally fabrication of CeO2@Xanthan NCs, (Figure 1).
Thus, in continuation of our works on biosynthesis of nanostructures and their applications to solving the environmental challenges, Through this research, for the first time the Allium tricoccum extract was used as an antioxidant source to biological synthesis of CeO2@Xanthan NCs through a one pot, simple, green and cost-effective procedure. The catalytic capability of bioactive NCs was assessed to the reduction [5] of 4-NP to 4-AP and also removal of aromatics including PAHs at ambient temperature.
Preparation of the Allium Tricoccum Extract
50 g of powdered root of the plant was mixed to 300 ml distilled water for 30 minutes at 85 °C. The extract was filtered to use as antioxidant reducing source [6].
One Pot Green Synthesis of CeO2@Xanthan NCs
100 ml of 0.008 M Ce(OH)3 solution was added to 200 ml of Allium tricoccum extract containing 3.0 g natural xanthan under reflux condition at 80 ºC while stirring for 2 hours at [7] pH 10 (as adjusted by NaOH) until formation a light black precipitate of CeO2@Xanthan NCs as then monitored using the identification techniques. The precipitate then was separated using filtration and washed with hot ethanol for many times to remove impurities. The clean precipitate then was dried at room temperature to use as nanocatalyst.
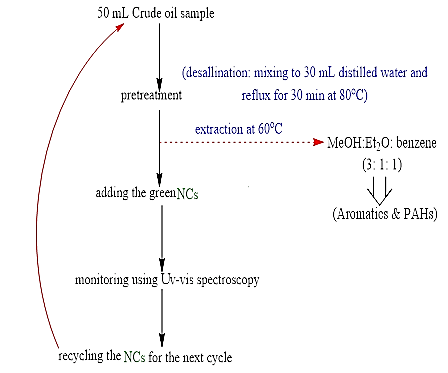
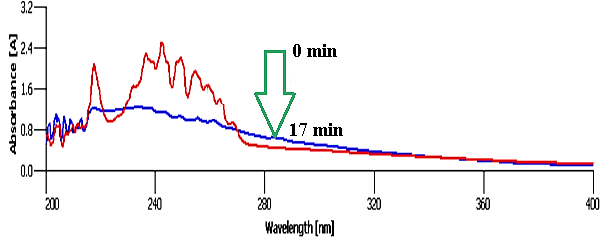
Adsorption of Aromatics and PAHs from Crude Oil Using CeO2@Xanthan NCsH: The adsorption of aromatics including PAHs from model crude oil (as model system) using the green synthesized NCs was as steps shown in scheme 1. After extracting the aromatics using NCs, both the rest of the crude oil sample was introduced to Uv-vis spectroscopy to study the removal of aromatics [8]. Then, the nanocatalyst was separated and recovered to use in the next cycle of the removal process (Figure 2).
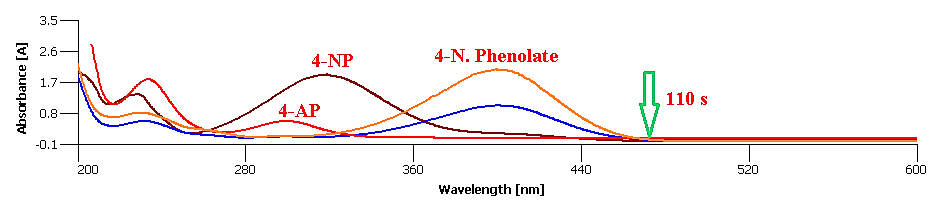
Reduction of the 4-NP by Using Green Nanocomposite: To study the catalytic activity of the prepared nanocatalyst [9], 10.0 mg of the CeO2@Xanthan NCs and 25 ml of 2.5 mM aqueous solution of 4-NP were mixed in a beaker at ambient temperature under stirring for 2 min. After addition of the newly prepared aqueous NaBH4 solution (5.3 × 10-3 M), the reduction process was monitored by the decrease of the absorption band at 400m using UV-Vis absorption spectroscopy [10]. The catalyst was separated after color fading of solution and recycled to use in the next reaction.
Results and Discussion
Spectrophotometric Analysis of Plant Extract
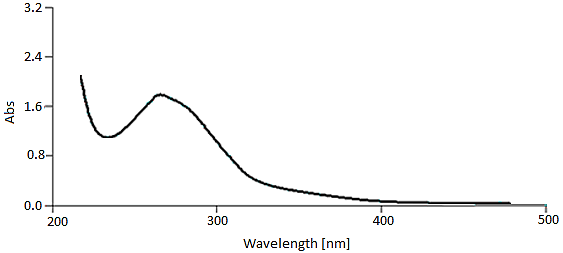
Figure 2 showed the signals at 263 nm (bond I) and 222 nm (bond II) due to the cinnamoyl and benzoyl systems of phenolic compounds as the main constituents of the [11] plant phytochemicals, respectively (Figure 3).
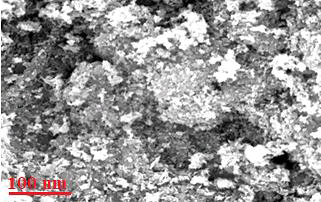
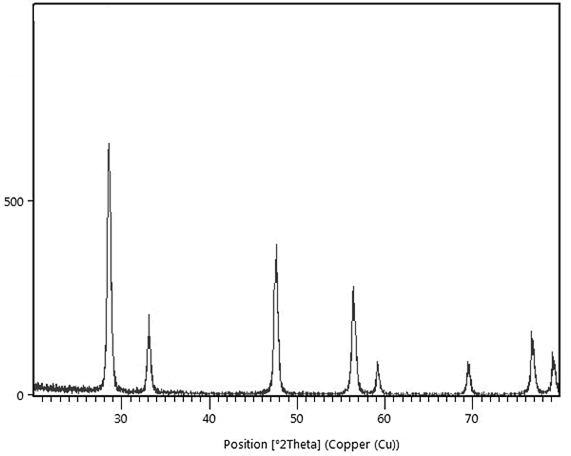
Characterization of green synthesized CeO2@Xanthan NCs: Figure 3 shows FE-SEM micrograph of green nanocomposite [12]. They clearly indicated that the CeO2 NPs are well coated on the xanthan surface to fabricate the nanocomposite. Also based on the micrographs, the nanoparticles unifor mly distributed on the surface of the xanthan substrate in [13] a spherical shape and homogeneous morphology. For further confirmation concerning the biosynthesis of NCs, elemental mapping analysis was applied. The XRD analysis of nanocomposite shows its crystallinity and nonorange. Both [14] techniques again strongly show the fabrication of nanocomposite using the green method, (Figures 4, 5).
Catalytic ability of the green ncs for removal of crude oil aromatics: The potential of NCs was assessed for removal of aromatic compounds as highly toxic compounds for human life and ecosystem in real sample of crude oil. As Table 1 indicted [15] the optimization of the NCs amount, the best result was 7 mg of the catalyst (entry 7) which indicates the efficiency of NCs in this process mainly for presence of bioactive phytochemicals on its surface. The yield of the reaction doesn’t change by using more amount than 7 mg as the optimum amount of the catalyst for the reaction, (entry 8). Also [16], the best result for ability of Xanthan substrate solely (entry 4) in the process was 71 min which less amounts leads to a few adsorptions even after 100 min (entry 1). Also, the application of 3 mg of catalyst shows no considerable adsorption [17] signal even after 25 min (entry 5). Finally, as Table 1 shows a reverse relation between the adsorption time and the amount of the catalyst.
Table 1: Catalytic adsorption of aromatics
| Entry | Catalyst | Crude oil aromatics ( ml) | Catalyst (mg) | Time |
|---|---|---|---|---|
| 1 | Xanthan | 10 ml | 5 | 100 min* |
| 2 | Xanthan | 10 ml | 10 | 90 min* |
| 3 | Xanthan | 10 ml | 15 | 72 min |
| 4 | Xanthan | 10 ml | 20 | 71 min |
| 5 | CeO2@Xanthan NCs | 10 ml | 3 | 25 min* |
| 6 | CeO2@Xanthan NCs | 10 ml | 5 | 22 min |
| 7 | CeO2@Xanthan NCs | 10 ml | 7 | 17 min |
| 8 | CeO2@Xanthan NCs | 10 ml | 10 | 17 min |
Note: *: a few adsorbed
According the Table 1 and Figure 6, the application of xanthan substrate in different amounts has no considerable effect on the removal of aromatics even after passing a lot of times, (entries 1 to 4). After using the NCs in the process, removal of the aromatics was occurred which this fact refers to the presence [18] of CeO2 NPs and also the ability of phytochemicals on the surface of NCs as capping agent which enhance the synergistic effect of the catalyst via the large surface area (reaction) of the NCs. These all parameters caused to efficiency of the NCs in removal of aromatics and PAHs. The best result obtained by the application of the NCs in the mentioned reaction was 7 mg, (Table 1, entry 6), which as depicted in (figure 6), this amount removed all aromatics during 17 min.
Evaluation of the Catalytic Activity of the CeO2@Xanthan NCs through the Reduction of 4-NP
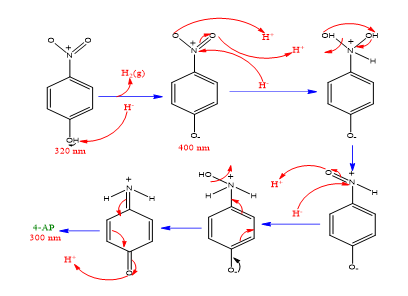
Degradation of the 4-NP was performed using NaBH4 and CeO2@Xanthan NCs as reducing agent and catalyst, respectively. In the mentioned process, no reaction was performed without using catalyst over a long time (Table 1, entry 1), which revealed the necessity of catalyst [19] presence for the reaction. The 4-NP solution shows one absorption peak at 317 nm, (Figures 7,8). Important factors affecting catalytic reduction of 4-NP such as concentration of NaBH4 and catalyst loading were investigated and optimized (Table 1). Without using NaBH4, the reduction reaction was not completed (Table 1, entry 2). After the addition of the NaBH4 to 4-NP solution, the color of the solution changes due to the formation of the 4-nitrophenolate ions, Scheme 2. The observed absorption peak at 400 nm confirms the formation of the 4-nitrophenolate ions which in the absence of the catalyst it remains unaltered even after passing a lot of times (Table 1, entry 1). After the addition of the catalyst to the 4-NP+NaBH4 mixture, the absorption maxima of the 4-nitrophenolate ion at 400 decreases and a new peak at 291 nm is appeared due to the formation of the 4-aminophenol (4-AP). Two amounts of catalyst (5.0 and 10.0 mg) and various concentrations of NaBH4 (50, 80 and 100 equivalents) were used in different experiments and the best result was obtained with 10.0mg of the catalyst and 80 equivalents of the NaBH4 (Table 1, entry 6). Increasing the amount of NaBH4 didn’t have any effect on reducing the reaction time (Table 1, entry 7). Also [20], the catalytic performance of the CeO2@Xanthan NCs was compared with CeO2 NPs and Xanthan substrate and the results are presented in Table 1. The completion of the reaction by Xanthan was longer than when green Nano composite was used (Table 1, entry 8) due to the positive effect of the CeO2 NPs through providing high surface area and also adsorbed phytochemicals on the surface of the NCs. The higher catalytic efficiency of NCs in comparison with CeO2 NPs (Table 1, entry 9) was attributed to the synergistic effect of xanthan as support which play an important role [21] in the catalyst structure by preventing the agglomeration of the CeO2 NPs (Table 2).
Table 2: Effect of the catalyst loading and concentration of NaBH4 in the reduction of 4-NP to 4-AP.
| Entry | Catalyst (mg) | NaBH4 (equivalents) | Time |
|---|---|---|---|
| 1 | - | 100 | 120 mina |
| 2 | CeO2@Xanthan NCs (10.0) | 0 | 100 minb |
| 3 | CeO2@Xanthan NCs (5.0) | 50 | 40 minb |
| 4 | CeO2@Xanthan NCs (5.0) | 80 | 200 s |
| 5 | CeO2@Xanthan NCs (5.0) | 100 | 120 s |
| 6 | CeO2@Xanthan NCs (10.0) | 80 | 110 s |
| 7 | CeO2@Xanthan NCs (10.0) | 100 | 110 s |
| 8 | Xanthan (10.0) | 100 | 65 mina |
| 9 | CeO2 NPs (5.0) | 100 | 25 min |
aNo reaction. bNot completed.
Conclusion
During this study, the natural Xanthan was used as the substrate for fabrication of CeO2@Xanthan NCs using the antioxidant potential of Allium tricoccum extract to act as a catalyst in a series of ecofriendly reactions. The green nanocatalyst was absolutely structurally elucidated using SEM and XRD techniques. Application of the natural Xanthan substrate and also adsorption of plant phytochemicals showed a very good effect on the catalytic activity and efficiency of the nanocatalyst for the removal of crude oil aromatics and conversion reaction of the toxic 4-NP to the safe 4-AP compound in ambient temperature. Furthermore, the green catalyst demonstrated a high potential of recycling for the mentioned reactions which indicates its ability as efficient catalyst to use in related industries.
Acknowledgements
We gratefully acknowledge from the Homayoun Sugar Factory for the support of this work.
References
- Toigo M, Boutellier U. Eur J Appl Physiol. 2006;97:643-663.
- Sendl A, Elbl G, Steinke B, et al. Planta Med. 1992;58(1):1-7.
- Sobolewska D, Podolak I, Makowska J, et al. Phytochem Rev. 2015;14:81-97.
- Nazarahari JM, Manshad KA, Moradi S, et al. Nanomaterials. 2020;10:2280.
- Omidi A, Manshad AK, Moradi S et al. J Mol Liq. 2020;316:113880.
- Hamad SM, Mahmud SA, Sajadi SM, et al. Surfaces Interfaces. 2019;15:125-134.
- Mahmud SA. Int j chem biochem 2016;10:37-39.
- Mahmud SA. Moroc J Chem. 2017;5:5-4.
- Kiamehr M, Alipour B, Nasrollahzadeh M, et al. IET Nanobiotechnology. 2018;12:217-222.
- Bahari S, Sajadi SM. Arab J Chem. 2017;10:700-704.
- Sajadi SM, Maham M. J Chem Res. 2013;37:623-625.
- Joseph T, Kumar KV, Ramaswamy AV, et al. Catal Commun. 2007;8:629-634.
- Obed SA, Mohammed PA. Cihan University Erbil Scientific J. 2021;5:24-31.
- Maryami M, Nasrollahzadeh M, Sajadi SM. Sep Purif Technol. 2017;184:298-307.
- Sajadi SM, Kadir DH, Balaky SM, et al. Surfaces Interfaces. 2021;23:100908.
- Kadir DH. Food Sci Nutr. 2021;9:3491-3499.
- Hosseini S, Nasiri P, Kadir DH, et al. arXiv preprint arXiv. 2017;1711:01542.
- Mohammed AJ, Hassan MM, Kadir DH et al. Int. J Adv Trends Comput eng. 2020;9:3161-3172.
- Othman GQ, Saeed RS, Kadir DH, et al. J Phys Conf Ser. 2019;1294:062110.
- Melendez Torres GJ, Kagan SH. Int J Older People Nurs. 2016;11:83-84.
- Nasrollahzadeh M, Sajadi SM. Ceramics Int. 2015;41:14435-14439.